Instructions for authors
- Page Path
-
- HOME
- FOR CONTRIBUTORS
- Instructions for authors


|
|
|
The Clinical and Molecular Hepatology publishes original basic and clinical research on liver diseases. Manuscripts should be submitted electronically (https://mc04.manuscriptcentral.com/cmh). The journal is published in English on 1st in January, April, July,
and October.
Authors lacking ability with English syntax should seek the appropriate editorial assistance prior to submitting their manuscripts.
These guidelines are in accordance with the ŌĆ£Uniform Requirements for Manuscripts Submitted to Biomedical Journals,ŌĆØ published by the International Committee of Medical Journal Editors at http://www.icmje.org. The Editorial Office, the Clinical and Molecular Hepatology Room A1210, Mapo Trapalace, 53 Mapo-daero, Mapo-gu, 04158, Seoul, Korea Tel.: 82-2-703-0051, Fax: 82-2-703-0071, E-mail: kasl@kams.or.kr
Contributions may be submitted as original articles, review articles, editorials and special topics. Special topics cover guidelines, meeting reports and hepatology issues elsewhere. Review articles, editorials and special topics are invited by the editorial board. However, authors who are interested in contributing reviews can submit reviews and are subjected to peer review. Letters to the editor may be subjected to peer review and undergo editing for clarity and brevity.
All investigations involving human participants must be conducted according to the ethical guidelines of the Declaration of Helsinki, and be approved by the institutional review board. For studies involving animal experimentation, author(s) must provide assurance that all the animals received humane care according to the criteria outlined in the NIH "Guide for the Care and Use of Laboratory Animals". The author must state that the use of animals (means all mammals and birds) in the manuscript was approved by the institutional Animal Ethical Committee (AEC) in accordance to the article 14th of Korean Animal Protection Law, or equivalent, in the paper. It must be clearly stated that animal use has complied to the article 13th of Korean Animal Protection Law (The principles of animal use) and the relevant institutional polices in the manuscript. Copies of the protocol approved by institutional AEC or equivalents, must be available for review by the editor if necessary.
The corresponding author must give written assurance that neither the submitted material nor portions thereof have been published previously or are under consideration for publication elsewhere. Any material that could constitute prior or concurrent publication of similar data by any one of the authors should be submitted with the manuscript. It is assumed that the corresponding author speaks for his or her co-authors and certifies that all the listed authors meaningfully participated in the study and that they have seen and approved the final manuscript. Authors should acknowledge any commercial affiliation or consultancy that could be constructed as potential conflicts of interest under a heading ŌĆ£Conflict of Interest statementŌĆØ prior to the references. For the policies on the research and publication ethics not stated in this instructions, ŌĆśGood Publication Practice Guidelines for Medical Journals ( https://www.kamje.or.kr/board/view?b_name=bo_publication&bo_id=7&per_page= )ŌĆÖor ŌĆśGuidelines on good publication ( http://www.publicationethics.org.uk/guidelines )ŌĆÖ can be applied. Ensure correct use of the terms sex (when reporting biological factors) and gender (Identity, psychosocial or cultureral factors), and, unless inappropriate, report the sex and/or gender of study participants, the sex of animals or cells, and describe the methods used to determine sex and gender. If the study was done involving an exclusive population, for example in only one sex, authors should justify why, except in obvious cases, (e.g., prostate cancer). Authors should define how they determined race or ethnicity and justify their relevance.
The manuscript should be written in A4 (21├Ś30 cm) paper in double space texts by leaving 3 cm space in the right, left, top and bottom sides at 10 point fonts.
Original articles Original articles describing clinical and basic studies in the field of hepatology. Manuscripts are expected to be well-organized and clearly written. They should not exceed 6,000 words, including the abstract, references, tables, and figure legends. No more than 8 figures and tables, with a maximum of 6 panels per figure. It is permitted for you to submit additional methodological details, non-essential figures or portions of your manuscript as supplementary material for online publication only. References cited in the main text may not be listed in the supplementary materials. The only references be listed in the supplement are those cited exclusively in the supplement. References should not exceed a maximum of 50. Original article must arranged as follows: (1) title page (2) abstract (250 words or less with a list of 5 or less key words), (3) introduction, (4) materials and methods (or patients and methods), (5) results, (6) discussion, (7) acknowledgements, (8) conflict of interest statement (9) references, (10) tables, and (11) figure legends. In case of submission of original articles (not applicable for reviews, editorials, and letters), authors should summarize the contents of the article in a concise, pictorial form designed to easily understand main findings of the work described in the article. Graphical abstracts should be submitted as a separate JPG or TIFF files at the online submission step of file upload. The submission of the graphical abstract is mandatory when submitting an original article. Graphical abstracts should be provided as an image with a minimum size of 531 ├Ś 531 pixels (height ├Ś width) using a minimum resolution of 600 dpi. When submitting a larger image, please make sure to use the same ratio. Also, please note that your image will be scaled proportionally to fit in the available window, which is a rectangle with a size of 200 ├Ś 500 pixels. Review articles Review articles on selected topics of interest for the readers of the Clinical and Molecular Hepatology and will be solicited by the Editors. Review articles are expected to be clear, concise and updated. The maximum length is 5,000 words. The inclusion of a maximum of 8 high quality tables and/or colored figures to summarize critical points is highly desirable. Editorials This section consists of invited brief editorial comments on articles published in the Clinical and Molecular Hepatology. The length of an editorial should not exceed 1,500 words and 1 table or 1 figure is allowed. References should not exceed a maximum of 20. Letters to the editor Letters to the editor should be related to a recent article published in the Clinical and Molecular Hepatology within previous two years. Letters to the editor must arranged as follows: (1) title page, (2) body (3) references (maximum of 15), and (4) a maximum number of 1 tables or figures is allowed. The length of an letter to the editor should not exceed 800 words, and the maximum number of authors is 6. Abstract is not required. Correspondence The correspondence consists of replies on editorials from the authors of the original publication in the Clinical and Molecular Hepatology. The length of an correspondence should not exceed 1,500 words and 1 table or 1 figure is allowed. References should not exceed a maximum of 15. Correspondence letters are not usually peer reviewed, but we might invite replies from the authors of the original publication. Special topics Special topics should be no longer than 800 words (including references) and 10 or less references. Snapshot Snapshot consists of a large single page figure with schematic diagrams and tables that graphically summarize current knowledge about a particular subject within the field of hepatology. A detailed figure legend which includes all relevant information can be included and may be incorporated into the main figure. The figure is accompanied by a short summary article that should not exceed a maximum of 600 words. References should not exceed a maximum of 10. The snapshot should contain a descriptive title. 1. Title page
Provide a concise title. List the full names of all authors and their institutional affiliation. In a multi-authored work involving more than a single institution, indicate individual affiliation by means of superscript Arabic numbers. Indicate a change of address in a similar fashion. List the footnotes to the title page. Provide the contact information for the corresponding author (name, address, telephone number, fax number, e-mail address and Orcid ID), and running title (Less than 50 characters). All abbreviations should be explained in this page (e.g. AFP, alpha fetoprotein; ALT, alanine aminotransferase). The Clinical and Molecular Hepatology employs a system to screen plagiarism (CrossRef). When submitting your manuscript to this journal, you accept that your manuscript may be screened for plagiarism against previously published material.
2. Abstract
Abstract of original articles must contain 250 words or less and must be organized as follows: Background/Aims, Methods, Results, and Conclusions. Three to Five keywords should be provided at the end of the abstract.
3. Highlight
Authors of original articles are requested to include ŌĆ£HighlightsŌĆØ which consist of three to four sentences summarizing the originality and main findings of the article. ŌĆ£HighlightsŌĆØ should not exceed 100 words in total. Highlights must be organized in a box and placed after the end of the abstract. The authors are encouraged to include the ŌĆ£HighlightsŌĆØ with initial article submission. When submitting a revised manuscript, the submission of the ŌĆ£HighlightsŌĆØ is mandatory.
4. Introduction
Provide the minimum background information that will orient the general reader. Do not engage in a literature review.
5. Methods
Provide a level of detail such that another investigator could repeat the work. For methods that are used without significant modification, citation of the original work will suffice. Identify and provide references for all the statistical methods used.
6. Results and Discussion
Present the major findings of the study in graphical form if practicable. Do not illustrate minor details if their message is adequately conveyed by simple descriptive text. Mention all the tables and figures. In the discussion, concisely present the implications of the new findings for the field as a whole, minimizing any reiteration of the results and avoid repetition of material in the introduction; keeping a close focus on the specific topic of the paper.
7. Acknowledgements
An acknowledgement of persons who made a genuine assistance and provided special reagents may be included. Grant and financial support related with the work should be specifically stated.
8. AuthorsŌĆÖ contribution
Based on the ICMJE guidelines for authorship criteria, how each author has contributed to the paper should be clarified (e.g, Conception or design of the work, Data collection, Data analysis and interpretation, Drafting the article, Critical revision of the article, and Final approval of the version to be published).
9. References
References should be numbered in the order they are cited, and the number of reference should be marked in the text by means of a superscript Arabic numerical. Only literature that is published or in press (with the name of the publication) may be numbered and listed; abstracts and letters to the editor may be cited, but they must be less than 3 years old and identified as such. Cite the names of all authors when there are six or less; when seven or more list the first six followed by et al.
Articles in journals
1. Kim YS, Jung ES, Hur W, Bae SH, Choi JY, Song MJ, et al. Noninvasive predictors of nonalcoholic steatohepatitis in Korean patients with histologically proven nonalcoholic fatty liver disease. Clin Mol Hepatol 2013;19:120-130. 2. Chung C, Iwakiri Y. The lymphatic vascular system in liver diseases: its role in ascites formation. Clin Mol Hepatol 2013;19:99-104. Literature in press An online article that has not yet been published in an issue can be cited by its Digital Object Identifier (DOI). The DOI will remain valid and allow an article to be tracked even after its allocation to an issue. Wong GL. Management of chronic hepatitis B patients in immunetolerant phase: what latestguidelines recommend. Clin Mol Hepatol. 2018Jan 22. doi: 10.3350/cmh.2017.0068. Book chapters 1. Gumucio JJ, Berkowitz CM. Structural organization of the liver and function of the hepatic acinus. In: Kaplowitz N, ed. Liver and Biliary Diseases. Vol I. 2nd ed. Baltimore: Williams & Wilkins, 1992:2-17. Abstract or Article in a supplement 1. Cho YJ, Lee SH, Kim BH, Yang SK, Jo YH, Lee DH. Characteristics of hepatocellular carcinoma with reference to ages in Korean patients [Abstract]. Hepatology 1998;28:246A. 2. Bellentani S, Marino M. Epidemiology and natural history of non-alcoholic fatty liver disease (NAFLD). Ann Hepatol 2009;8(Suppl 1):S4-S8. Websites 1. Ontario Chronic Disease Prevention Alliance (OCDPA). Economic cost of chronic disease in Canada 1995-2003. OCDPA web site, <http://www.ocdpa.on.ca/OCDPA/docs/OCDPA_EconomicCosts.pdf>. Accessed 7 Sep 2011. 10. Permissions
Direct quotations, tables or illustrations taken from copy-righted material must be accompanied by written permission for their use from the publisher. The permission is presented as a footnote or addition to the legend and it must provide complete information as to the source. Photographs of identifiable persons must be accompanied by a signed release that indicates their informed consent.
11. Abbreviations
Please include an alphabetical list of all non-standard abbreviations used within the manuscript. Please do not abbreviate unless a term is used more than five times in a paper. In this case, the abbreviation should be spelled out, in its first use in the text with the abbrevi-ated form in parentheses, and it should also be listed on the footnote page. Abbreviations used in figures or tables should be defined in the legend.
12. Drug names
Use generic names. The proprietary name may be mentioned in parenthesis. The names and locations (city and state or country) of manufacturers should be included in parentheses when mentioning proprietary drugs, tools, instruments, software, etc.
13. Tables
Prepare tables on individual sheets of paper, double spaced and numbered consecutively with Arabic numerals in the order of their appearance in the text. The title of tables should be written concisely in clauses and phrases. The first letter of the table title starts with a capital letter. Explain all abbreviations and symbols such as *, ŌĆĀ, ŌĆĪ, ┬¦, Ōłź, , **, ŌĆĀŌĆĀ, ŌĆĪŌĆĪ, ┬¦┬¦. Do not duplicate the material presented in a figure.
14. Figure legends
Number the figures with Arabic numerals in the order they are mentioned in the text. Provide a title (this should not appear on the figure itself) and sufficient explanation to render the figure intelligible without reference to the text. For any copyrighted material, indicate that permission has been obtained (see Permissions, above). Figure legends should be typed consecutively on a separate sheet of paper.
15. Figures
Illustrations should be sharp and clear. Figure files can be uploaded in the JPG or TIFF formats which authors prefer at a final resolution of not less than 300 dpi. Microscopic pictures should be explained according to the staining method and scaled by the power of magnification. Authors are charged for color figures.
The journal utilizes blind peer-review in evaluating manuscripts for publication. Submitted papers will be reviewed by at least two referees, and decisions will be available in approximately one months. With respect to the revision and resubmission of manuscripts, it is the journalŌĆÖs policy to allow a couple of resubmission only, which should be received within 2 months from the time of receipt of the initial review letter. In general, a manuscript requiring more than a couple of revision or returned beyond 2 months will be handled as a new submission. The journal does not have article submission charges.
As of January 1, 2022, the Clinical and Molecular Hepatology charges a publication fee of US$1,000 (KRW1,200,000) per accepted article and starting with articles submitted on February 5, 2024, the APC is $1,500 (KRW2,000,000). The authors will receive an invoice for APC shortly after the corrected proof of their accepted manuscript has been finalized. Please note that only ŌĆ£original articlesŌĆØ are subject to article processing charges.
A fast-track review process is available for authors who desire quick publication of their papers.
Fast-track manuscripts will be handled by the Editor in Chief, and the first decision following a full peer-review of the manuscript will be made within 7 days of submission. The accepted papers will be published within 2 weeks from the date of acceptance, in the next issue of the Clinical and Molecular Hepatology. An additional non-refundable processing fee (US$1,000) will be charged for the initiation of the fast-track process. A fast-track review does not guarantee acceptance. The journal is editorially independent and will assess your manuscript according to its own criteria. If your article is finally accepted, an article processing charge of US$1,000 will be additionally charged. If you wish to submit your article using the fast-track review process, please contact the Editorial Office in advance to arrange a peerreview process.
For the authors who wish to publish their paper as a cover page article, we offer full support in producing the illustration to go on the cover. The Clinical and Molecular Hepatology charges US$1,000 for the cover page illustration work. If you are interested, please contact the Editorial Office.
Copyright for all material published in the Clinical and Molecular Hepatology is vested in The Korean Association for the Study of the Liver. In accordance with the Copyright Act, all manuscripts must be accompanied by a Copyright transfer form signed by all authors and that follows these guidelines. Statements and opinions expressed in the articles and communications in the Clinical and Molecular Hepatology are those of the author(s) and do not necessarily reflect the opinions of the Editor(s) or publisher, and the Editor(s) and publisher disclaim any responsibility or liability for such material. Neither the Editor(s) nor the publisher guarantees, warrants or endorses any product or service advertised in the journal; nor do they guarantee any claim made by the manufacturer of such product or service
|
-

-

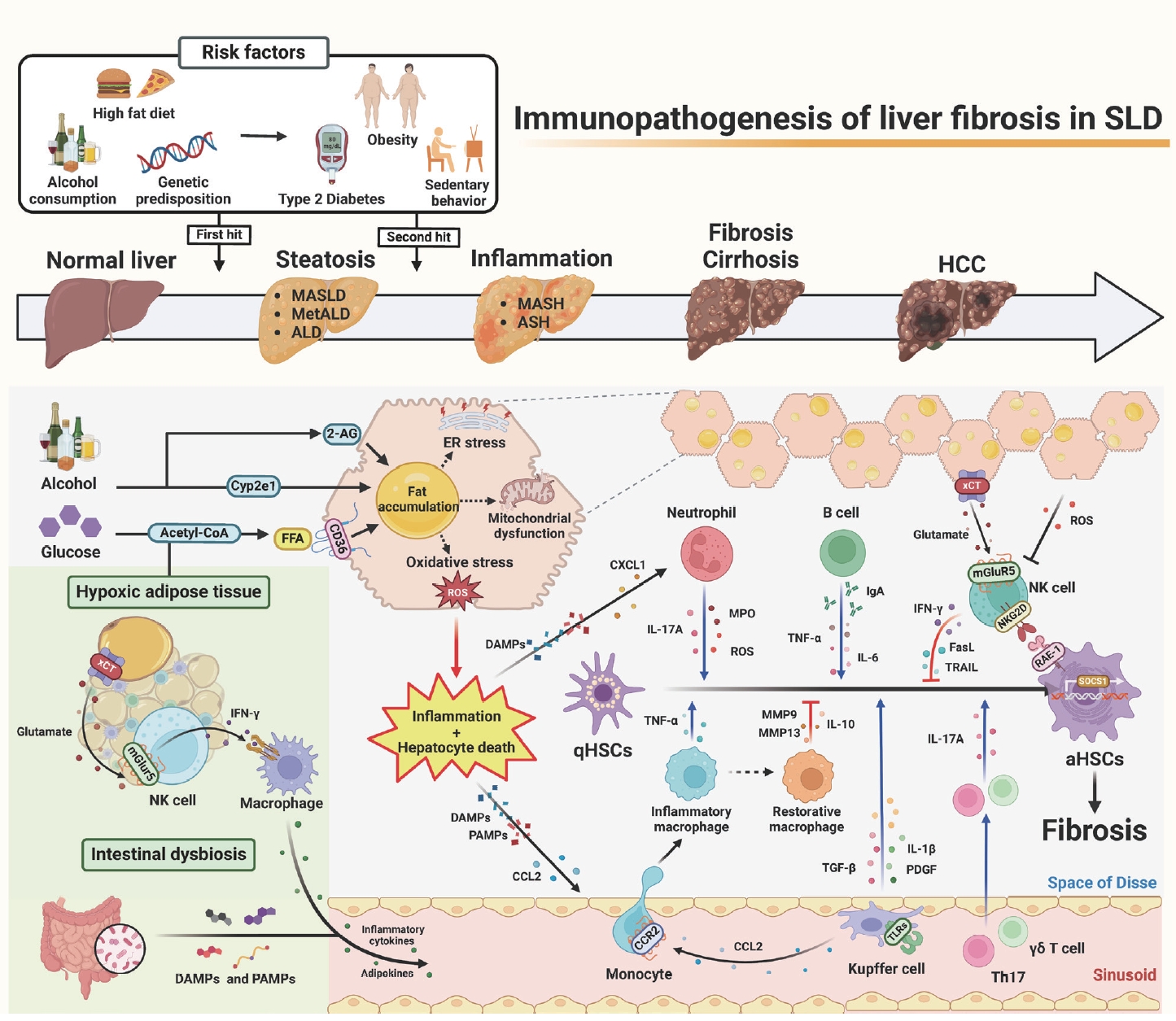
- Immunopathogenesis of liver fibrosis in steatotic liver disease
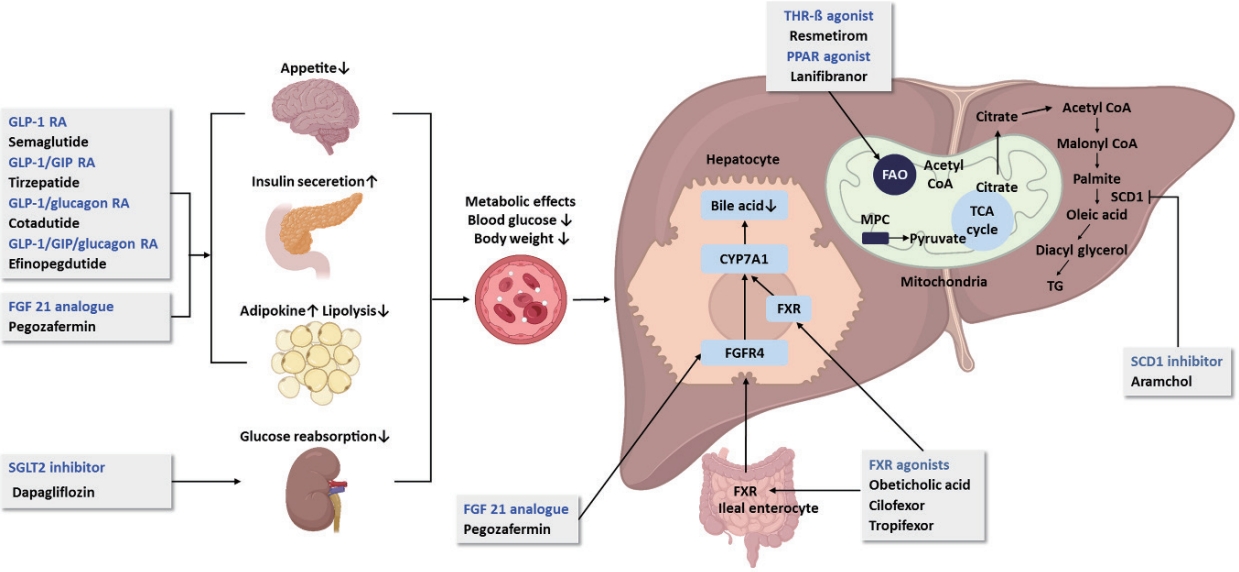
- Recent updates on pharmacologic therapy in non-alcoholic fatty liver disease
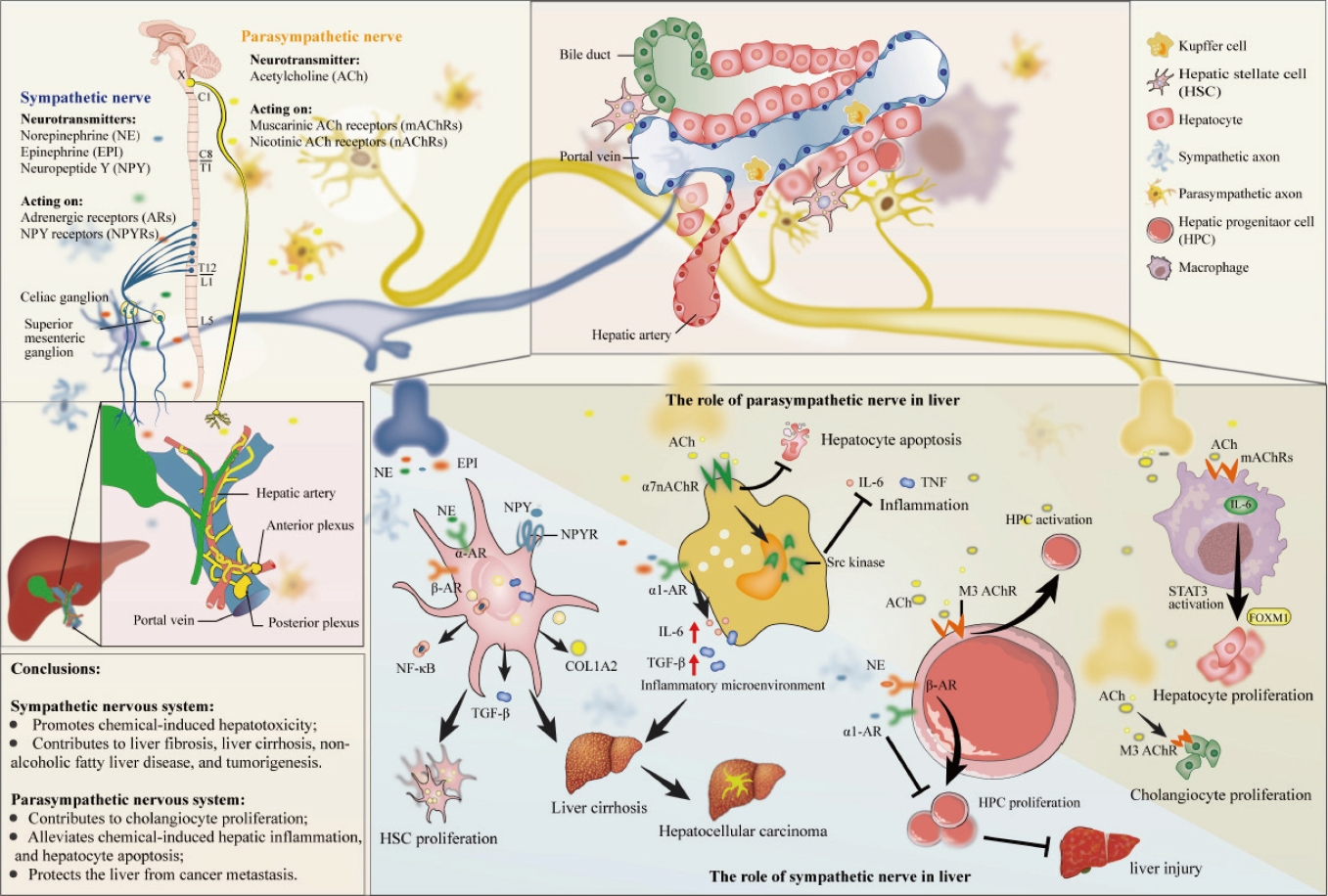
- The role of the hepatic autonomic nervous system
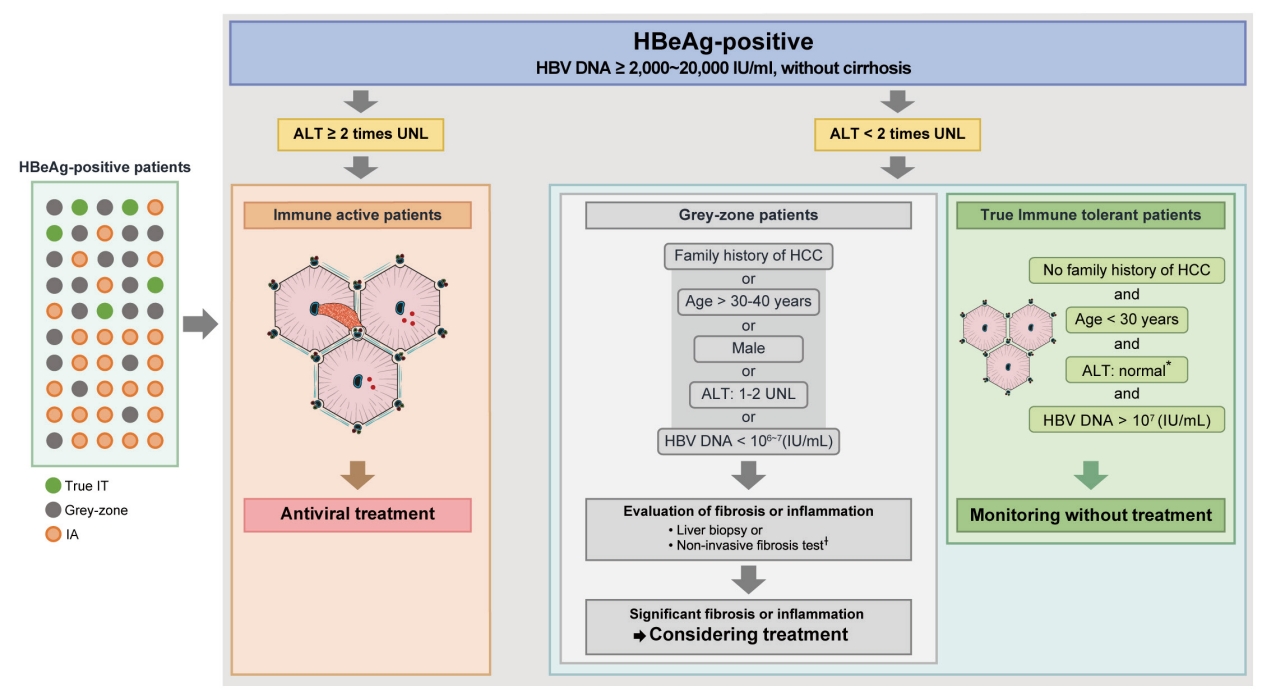
- HBeAg-positive grey-zone patients: Treatment beyond guideline recommendations?
- Systemic therapy in advanced hepatocellular carcinoma

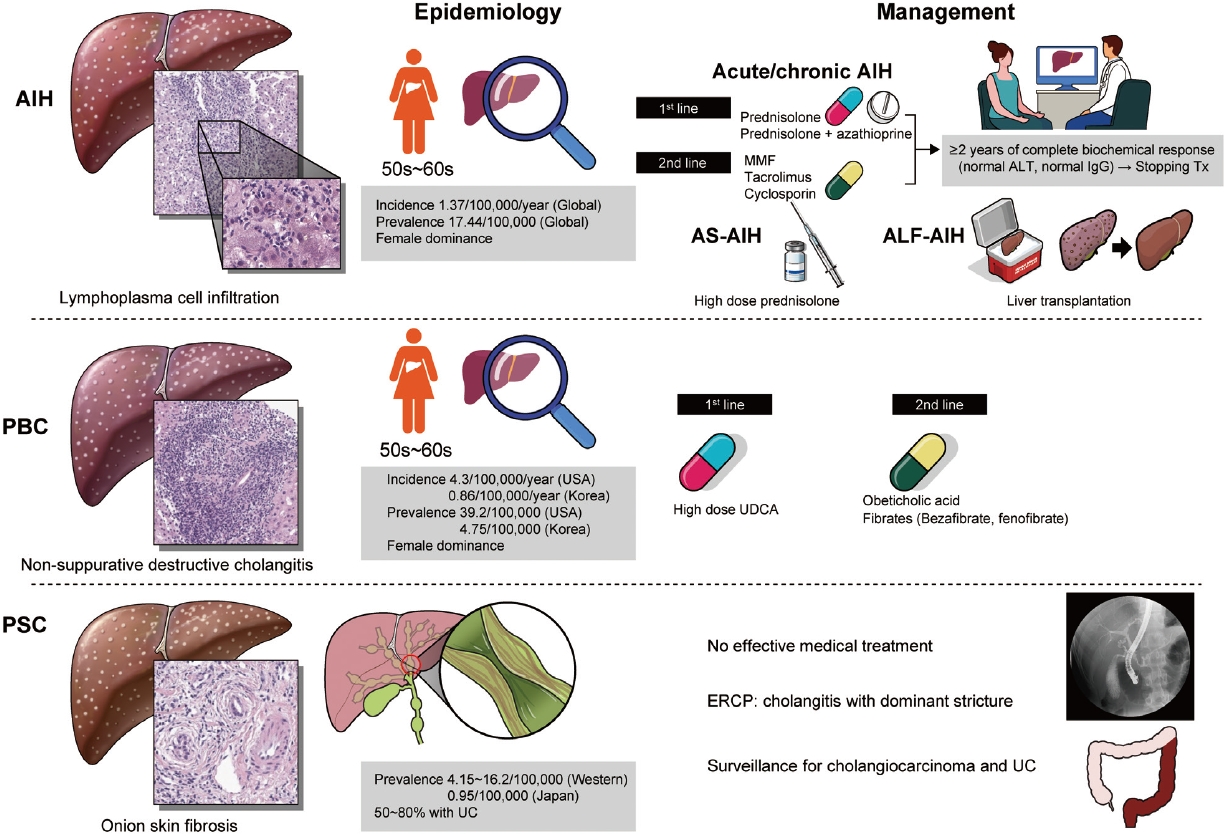
- Epidemiology and updated management for autoimmune liver disease
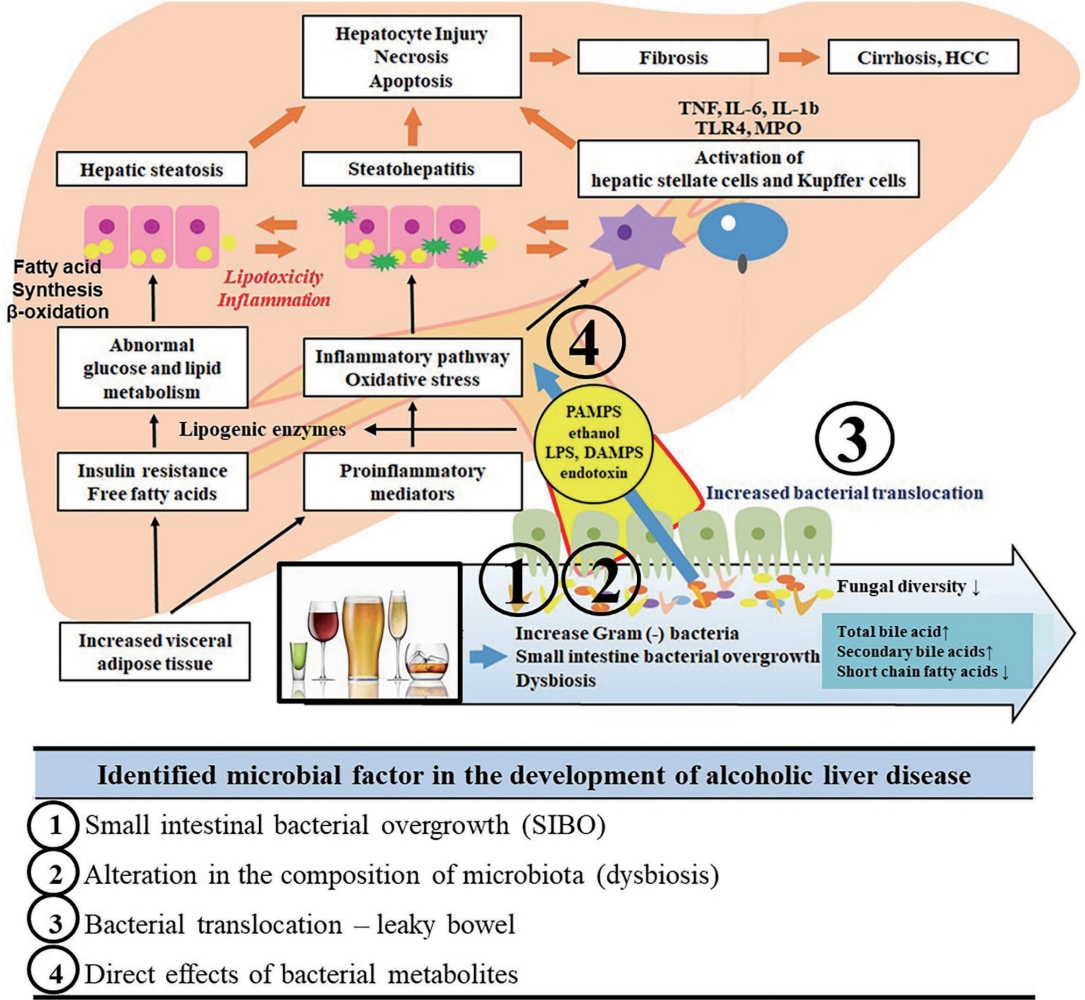
- Microbiome and metabolomics in alcoholic liver disease
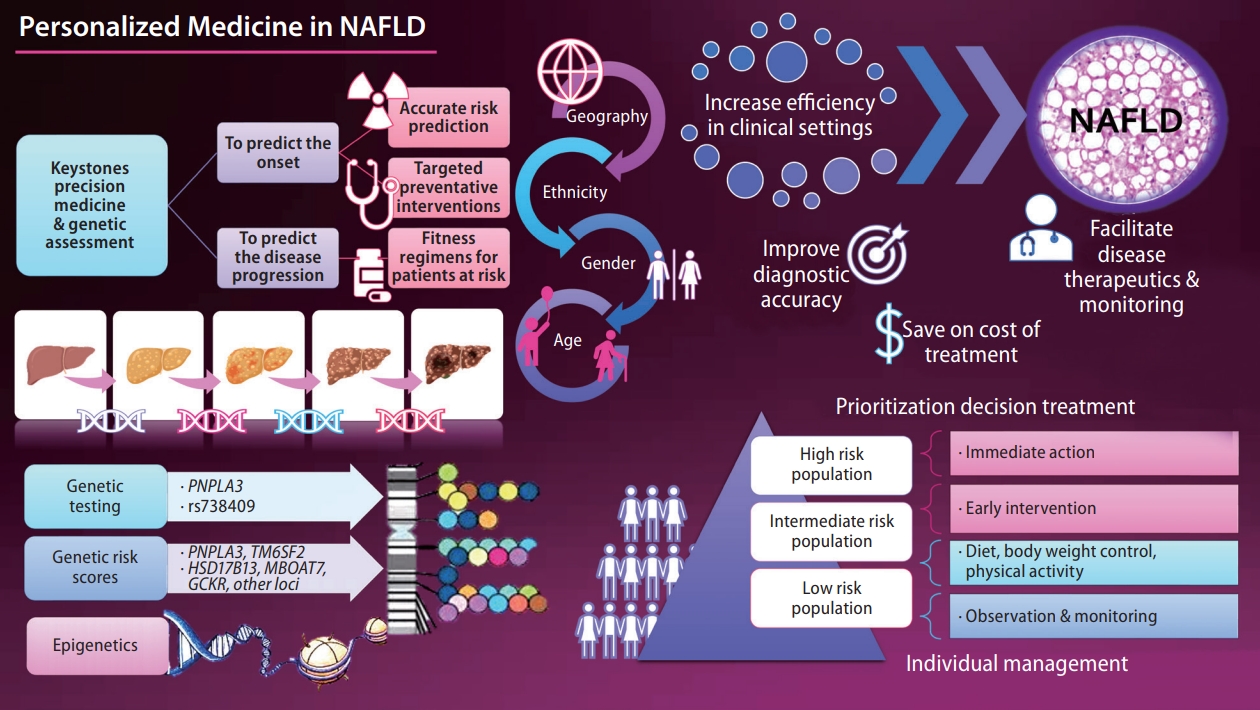
- Personalized medicine in nonalcoholic fatty liver disease
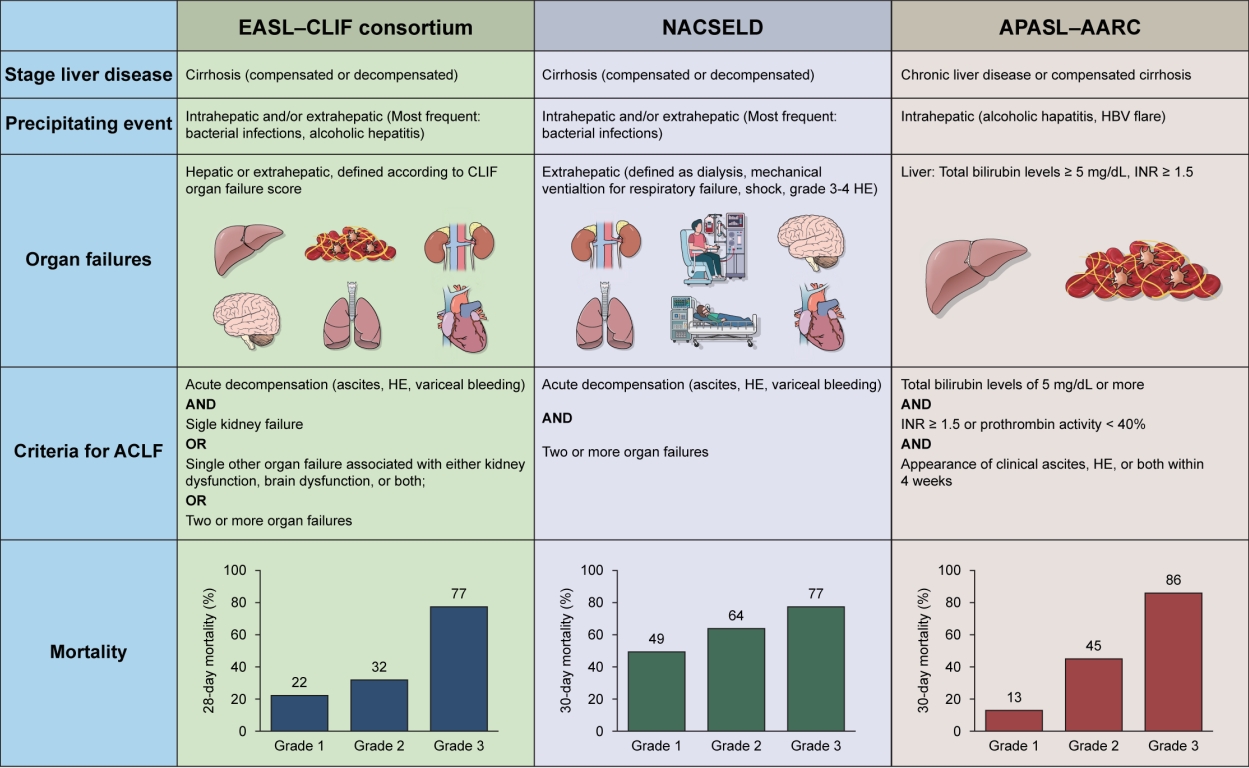
- Acute on chronic liver failure in cirrhosis
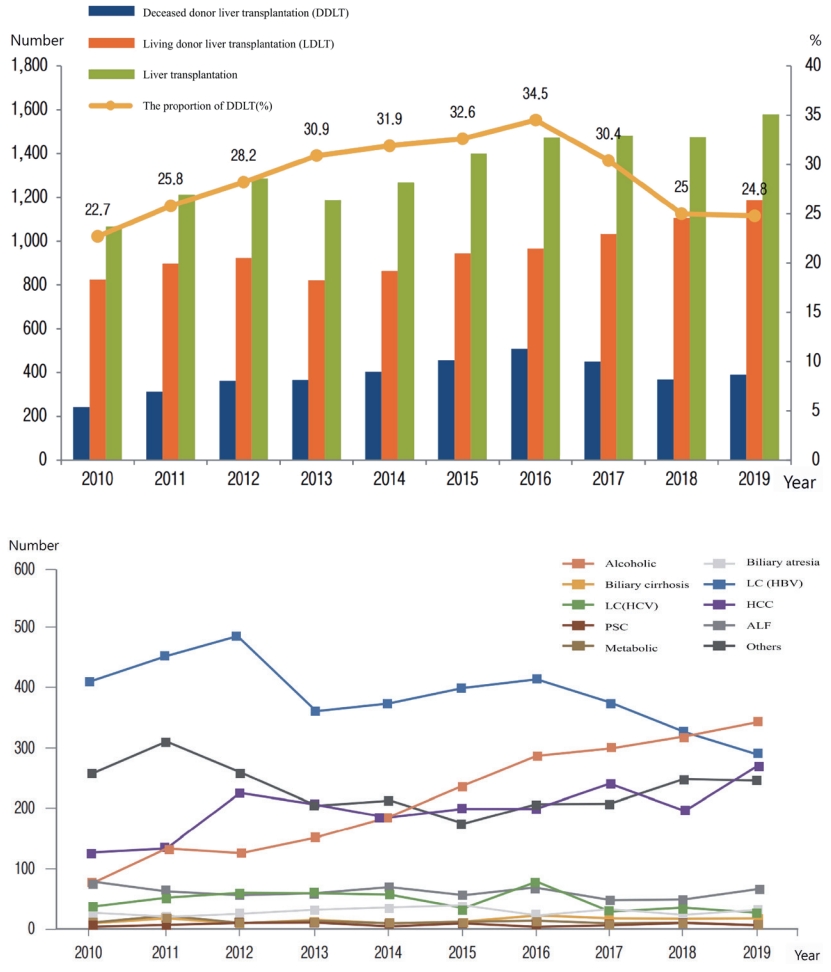
- Current status and outcome of liver transplantation in South Korea
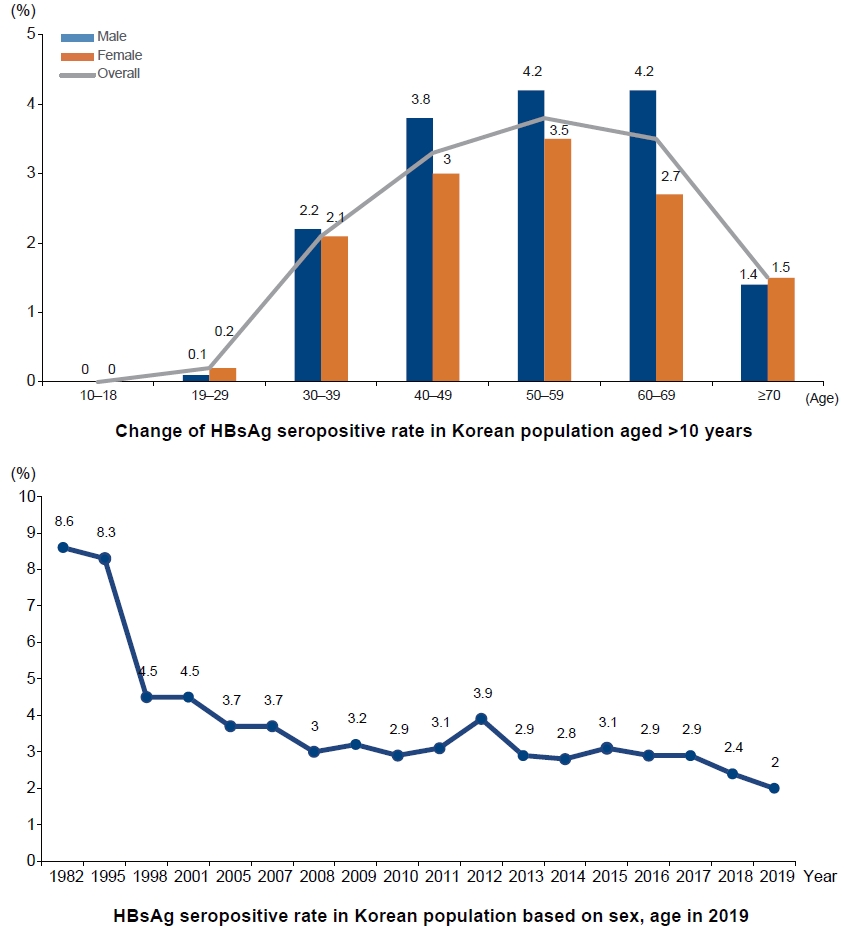
- History and future of hepatitis B virus control in South Korea
- Hepatocellular carcinoma statistics in South Korea


 Impact Factor8.9
Impact Factor8.9First decision
09 Days
Publication after acceptance
04 Days
*Last 12 months
- MOST VIEWED (Last 6 months)
- MOST CITED (2022-2024)







