1. Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet 2015;386:1546-1555.


2. Chae HB, Kim JH, Kim JK, Yim HJ. Current status of liver diseases in Korea: hepatitis B. The Korean Journal of Hepatology 2009;15 Suppl 6:S13-S24.


5. Hui CK, Leung N, Yuen ST, Zhang HY, Leung KW, Lu L, et al. Natural history and disease progression in Chinese chronic hepatitis B patients in immuneŌĆÉtolerant phase. Hepatology 2007;46:395-401.


6. Tran TT. Immune tolerant hepatitis B: a clinical dilemma. Gastroenterol Hepatol (NY) 2011;7:511-516.
7. Chu CM, Hung SJ, Lin J, Tai DI, Liaw YF. Natural history of hepatitis B e antigen to antibody seroconversion in patients with normal serum aminotransferase levels. Am J Med 2004;116:829-834.


9. Livingston SE, Simonetti JP, Bulkow LR, Homan CE, Snowball MM, Cagle HH, et al. Clearance of hepatitis B e antigen in patients with chronic hepatitis B and genotypes A, B, C, D, and F. Gastroenterology 2007;133:1452-1457.


11. Lee PI, Chang MH, Lee CY, Hsu HY, Chen JS, Chen PJ, et al. Changes of serum hepatitis B virus DNA and aminotransferase levels during the course of chronic hepatitis B virus infection in children. Hepatology 1990;12(4 Pt 1):657-660.


12. Lok AS, Lai CL. Acute exacerbations in Chinese patients with chronic hepatitis B virus (HBV) infection. Incidence, predisposing factors and etiology. J Hepatol 1990;10:29-34.


13. McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology 2009;49(5 Suppl):S45-S55.


14. Bortolotti F, Guido M, Bartolacci S, Cadrobbi P, Crivellaro C, Noventa F, et al. Chronic hepatitis B in children after e antigen seroclearance: final report of a 29-year longitudinal study. Hepatology 2006;43:556-562.


15. Hsu YS, Chien RN, Yeh CT, Sheen IS, Chiou HY, Chu CM, et al. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology 2002;35:1522-1527.


16. Lok AS, Lai CL, Wu PC, Leung EK, Lam TS. Spontaneous hepatitis B e antigen to antibody seroconversion and reversion in Chinese patients with chronic hepatitis B virus infection. Gastroenterology 1987;92:1839-1843.


17. Yim HJ, Lok AS. Natural history of chronic hepatitis B virus infection: what we knew in 1981 and what we know in 2005. Hepatology 2006;43(2 Suppl 1):S173-S181.


18. Sheen IS, Liaw YF, Tai DI, Chu CM. Hepatic decompensation associated with hepatitis B e antigen clearance in chronic type B hepatitis. Gastroenterology 1985;89:732-735.


19. Martinot-Peignoux M, Boyer N, Colombat M, Akremi R, Pham BN, Ollivier S, et al. Serum hepatitis B virus DNA levels and liver histology in inactive HBsAg carriers. J Hepatol 2002;36:543-546.


20. Zacharakis GH, Koskinas J, Kotsiou S, Papoutselis M, Tzara F, Vafeiadis N, et al. Natural history of chronic HBV infection: a cohort study with up to 12 years follow-up in North Greece (part of the Interreg I-II/EC-project). J Med Virol 2005;77:173-179.


21. de Franchis R, Meucci G, Vecchi M, Tatarella M, Colombo M, Del Ninno E, et al. The natural history of asymptomatic hepatitis B surface antigen carriers. Ann Intern Med 1993;118:191-194.


22. Fattovich G. Natural history and prognosis of hepatitis B. Semin Liver Dis 2003;23:47-58.


23. Chen YC, Sheen IS, Chu CM, Liaw YF. Prognosis following spontaneous HBsAg seroclearance in chronic hepatitis B patients with or without concurrent infection. Gastroenterology 2002;123:1084-1089.


24. Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology 2009;50:661-662.


25. Funk ML, Rosenberg DM, Lok AS. World-wide epidemiology of HBeAg-negative chronic hepatitis B and associated precore and core promoter variants. J Viral Hepat 2002;9:52-61.


28. Croagh CM, Bell SJ, Slavin J, Kong YX, Chen RY, Locarnini S, et al. Increasing hepatitis B viral load is associated with risk of significant liver fibrosis in HBeAg-negative but not HBeAg-positive chronic hepatitis B. Liver Int 2010;30:1115-1122.


29. Yoo BC, Park JW, Kim HJ, Lee DH, Cha YJ, Park SM. Precore and core promoter mutations of hepatitis B virus and hepatitis B e antigen-negative chronic hepatitis B in Korea. J Hepatol 2003;38:98-103.


30. Raimondo G, Allain JP, Brunetto MR, Buendia MA, Chen DS, Colombo M, et al. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J Hepatol 2008;49:652-657.


31. Chu CM, Liaw YF. HBsAg seroclearance in asymptomatic carriers of high endemic areas: appreciably high rates during a long-term follow-up. Hepatology 2007;45:1187-1192.


32. Liu J, Yang HI, Lee MH, Lu SN, Jen CL, Wang LY, et al. Incidence and determinants of spontaneous hepatitis B surface antigen seroclearance: a community-based follow-up study. Gastroenterology 2010;139:474-482.


33. Liaw YF, Sheen IS, Chen TJ, Chu CM, Pao CC. Incidence, determinants and significance of delayed clearance of serum HBsAg in chronic hepatitis B virus infection: a prospective study. Hepatology 1991;13:627-631.


35. Kuang XJ, Jia RR, Huo RR, Yu JJ, Wang JJ, Xiang BD, et al. Systematic review of risk factors of hepatocellular carcinoma after hepatitis B surface antigen seroclearance. J Viral Hepat 2018;25:1026-1037.


36. Yip TC, Chan HL, Wong VW, Tse YK, Lam KL, Wong GL. Impact of age and gender on risk of hepatocellular carcinoma after hepatitis B surface antigen seroclearance. J Hepatol 2017;67:902-908.


37. Lee KJ, Han KH, Chun JY, Moon YM, Lee SI, Park IS, et al. Natural history of chronic hepatitis type B throughout long-term follow-up. Korean J Gastroenterol 1997;29:343-351.
40. Tseng TC, Liu CJ, Yang HC, Chen CL, Yang WT, Tsai CS, et al. Higher proportion of viral basal core promoter mutant increases the risk of liver cirrhosis in hepatitis B carriers. Gut 2015;64:292-302.


41. Jang JW, Chun JY, Park YM, Shin SK, Yoo W, Kim SO, et al. Mutational complex genotype of the hepatitis B virus X /precore regions as a novel predictive marker for hepatocellular carcinoma. Cancer Sci 2012;103:296-304.


42. Kim JK, Chang HY, Lee JM, Baatarkhuu O, Yoon YJ, Park JY, et al. Specific mutations in the enhancer II/core promoter/precore regions of hepatitis B virus subgenotype C2 in Korean patients with hepatocellular carcinoma. J Med Virol 2009;81:1002-1008.


43. Kao JH, Chen PJ, Lai MY, Chen DS. Basal core promoter mutations of hepatitis B virus increase the risk of hepatocellular carcinoma in hepatitis B carriers. Gastroenterology 2003;124:327-334.


45. Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006;295:65-73.


46. Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 2004;127(5 Suppl 1):S35-S50.


47. Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ, et al. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology 2006;130:678-686.


48. Bravi F, Tavani A, Bosetti C, Boffetta P, La Vecchia C. Coffee and the risk of hepatocellular carcinoma and chronic liver disease: a systematic review and meta-analysis of prospective studies. Eur J Cancer Prev 2017;26:368-377.


53. Lee M, Chung GE, Lee JH, Oh S, Nam JY, Chang Y, et al. Antiplatelet therapy and the risk of hepatocellular carcinoma in chronic hepatitis B patients on antiviral treatment. Hepatology 2017;66:1556-1569.


55. Hsiang JC, Wong GL, Tse YK, Wong VW, Yip TC, Chan HL. Statin and the risk of hepatocellular carcinoma and death in a hospitalbased hepatitis B-infected population: a propensity score landmark analysis. J Hepatol 2015;63:1190-1197.


56. Kim G, Jang SY, Nam CM, Kang ES. Statin use and the risk of hepatocellular carcinoma in patients at high risk: a nationwide nested case-control study. J Hepatol 2018;68:476-484.


57. Pradelli D, Soranna D, Scotti L, Zambon A, Catapano A, Mancia G, et al. Statins and primary liver cancer: a meta-analysis of observational studies. Eur J Cancer Prev 2013;22:229-234.


58. Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology 2013;144:323-332.


60. Yang HI, Yuen MF, Chan HL, Han KH, Chen PJ, Kim DY, et al. Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): development and validation of a predictive score. Lancet Oncol 2011;12:568-574.


62. Jung KS, Kim SU, Song K, Park JY, Kim DY, Ahn SH, et al. Validation of hepatitis B virus-related hepatocellular carcinoma prediction models in the era of antiviral therapy. Hepatology 2015;62:1757-1766.


63. Papatheodoridis G, Dalekos G, Sypsa V, Yurdaydin C, Buti M, Goulis J, et al. PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J Hepatol 2016;64:800-806.


64. Kim MN, Hwang SG, Rim KS, Kim BK, Park JY, Kim DY, et al. Validation of PAGE-B model in Asian chronic hepatitis B patients receiving entecavir or tenofovir. Liver Int 2017;37:1788-1795.


65. Kim JH, Kim YD, Lee M, Jun BG, Kim TS, Suk KT, et al. Modified PAGE-B score predicts the risk of hepatocellular carcinoma in Asians with chronic hepatitis B on antiviral therapy. J Hepatol 2018;69:1066-1073.


69. Mast EE, Margolis HS, Fiore AE, Brink EW, Goldstein ST, Wang SA, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep 2005;54(RR-16):1-31.
70. Mast EE, Weinbaum CM, Fiore AE, Alter MJ, Bell BP, Finelli L, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep 2006;55(RR-16):1-33 quiz CE1-4.
71. U.S. Public Health Service. Updated U.S. Public Health Service Guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep 2001;50(RR-11):1-52.

72. Lok AS, Lai CL, Wu PC. Prevalence of isolated antibody to hepatitis B core antigen in an area endemic for hepatitis B virus infection: implications in hepatitis B vaccination programs. Hepatology 1988;8:766-770.


73. McMahon BJ, Parkinson AJ, Helminiak C, Wainwright RB, Bulkow L, Kellerman-Douglas A, et al. Response to hepatitis B vaccine of persons positive for antibody to hepatitis B core antigen. Gastroenterology 1992;103:590-594.


74. Keeffe EB. Hepatitis A and B superimposed on chronic liver disease: vaccine-preventable diseases. Trans Am Clin Climatol Assoc 2006;117:227-237 discussion 237-238.


75. Craig AS, Schaffner W. Prevention of hepatitis A with the hepatitis A vaccine. N Engl J Med 2004;350:476-481.


76. Beasley RP, Hwang LY, Lee GC, Lan CC, Roan CH, Huang FY, et al. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet 1983;2:1099-1102.


77. Eke AC, Eleje GU, Eke UA, Xia Y, Liu J. Hepatitis B immunoglobulin during pregnancy for prevention of mother-to-child transmission of hepatitis B virus. Cochrane Database Syst Rev 2017;2:CD008545.


79. Beasley RP, Stevens CE, Shiao IS, Meng HC. Evidence against breast-feeding as a mechanism for vertical transmission of hepatitis B. Lancet 1975;2:740-741.


80. Chevillotte G, Durbec JP, Gerolami A, Berthezene P, Bidart JM, Camatte R. Interaction between hepatitis b virus and alcohol consumption in liver cirrhosis. An epidemiologic study. Gastroenterology 1983;85:141-145.


81. Villa E, Rubbiani L, Barchi T, Ferretti I, Grisendi A, De Palma M, et al. Susceptibility of chronic symptomless HBsAg carriers to ethanolinduced hepatic damage. Lancet 1982;2:1243-1244.


84. Yu MW, Lin CL, Liu CJ, Yang SH, Tseng YL, Wu CF. Influence of metabolic risk factors on risk of hepatocellular carcinoma and liverrelated death in men with chronic hepatitis B: a large cohort study. Gastroenterology 2017;153:1006-1017 e5.


85. Wong GL, Chan HL, Yu Z, Chan AW, Choi PC, Chim AM, et al. Coincidental metabolic syndrome increases the risk of liver fibrosis progression in patients with chronic hepatitis B--a prospective cohort study with paired transient elastography examinations. Aliment Pharmacol Ther 2014;39:883-893.


86. Chan AW, Wong GL, Chan HY, Tong JH, Yu YH, Choi PC, et al. Concurrent fatty liver increases risk of hepatocellular carcinoma among patients with chronic hepatitis B. J Gastroenterol Hepatol 2017;32:667-676.


87. Kim JH, Sinn DH, Gwak GY, Kang W, Paik YH, Choi MS, et al. Insulin resistance and the risk of hepatocellular carcinoma in chronic hepatitis B patients. J Gastroenterol Hepatol 2017;32:1100-1106.


89. Lai CL, Lau JY, Yeoh EK, Chang WK, Lin HJ. Significance of isolated anti-HBc seropositivity by ELISA: implications and the role of radioimmunoassay. J Med Virol 1992;36:180-183.


90. S├Īnchez-Quijano A, Jauregui JI, Leal M, Pineda JA, Castilla A, Abad MA, et al. Hepatitis B virus occult infection in subjects with persistent isolated anti-HBc reactivity. J Hepatol 1993;17:288-293.


91. Raimondo G, Navarra G, Mondello S, Costantino L, Colloredo G, Cucinotta E, et al. Occult hepatitis B virus in liver tissue of individuals without hepatic disease. J Hepatol 2008;48:743-746.


97. European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370-398.


98. World Health Organization (WHO). Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis B Infection. Geneva: WHO; 2015. p. 38.
99. Perrillo RP, Lai CL, Liaw YF, Dienstag JL, Schiff ER, Schalm SW, et al. Predictors of HBeAg loss after lamivudine treatment for chronic hepatitis B. Hepatology 2002;36:186-194.


100. Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med 2002;137:1-10.


101. Shim JJ, Kim JW, Oh CH, Lee YR, Lee JS, Park SY, et al. Serum alanine aminotransferase level and liver-related mortality in patients with chronic hepatitis B: a large national cohort study. Liver Int 2018;38:1751-1759.


102. Kariv R, Leshno M, Beth-Or A, Strul H, Blendis L, Kokia E, et al. Reevaluation of serum alanine aminotransferase upper normal limit and its modulating factors in a large-scale population study. Liver Int 2006;26:445-450.


103. Pawlotsky JM. Hepatitis B virus (HBV) DNA assays (methods and practical use) and viral kinetics. J Hepatol 2003;39 Suppl 1:S31-S35.


104. Chu CM, Liaw YF. Genotype C hepatitis B virus infection is associated with a higher risk of reactivation of hepatitis B and progression to cirrhosis than genotype B: a longitudinal study of hepatitis B e antigen-positive patients with normal aminotransferase levels at baseline. J Hepatol 2005;43:411-417.


105. Sumi H, Yokosuka O, Seki N, Arai M, Imazeki F, Kurihara T, et al. Influence of hepatitis B virus genotypes on the progression of chronic type B liver disease. Hepatology 2003;37:19-26.


107. Lin CL, Kao JH. Natural history of acute and chronic hepatitis B: the role of HBV genotypes and mutants. Best Pract Res Clin Gastroenterol 2017;31:249-255.


108. Kao JH, Wu NH, Chen PJ, Lai MY, Chen DS. Hepatitis B genotypes and the response to interferon therapy. J Hepatol 2000;33:998-1002.


109. Keeffe EB, Dieterich DT, Han SH, Jacobson IM, Martin P, Schiff ER, et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol 2008;6:1315-1341 quiz 1286.


110. Cornberg M, Wong VW, Locarnini S, Brunetto M, Janssen HLA, Chan HL. The role of quantitative hepatitis B surface antigen revisited. J Hepatol 2017;66:398-411.


111. Nguyen T, Thompson AJ, Bowden S, Croagh C, Bell S, Desmond PV, et al. Hepatitis B surface antigen levels during the natural history of chronic hepatitis B: a perspective on Asia. J Hepatol 2010;52:508-513.


112. Suh SJ, Bae SI, Kim JH, Kang K, Yeon JE, Byun KS. Clinical implications of the titer of serum hepatitis B surface antigen during the natural history of hepatitis B virus infection. J Med Virol 2014;86:117-123.


113. Liu J, Yang HI, Lee MH, Jen CL, Batrla-Utermann R, Lu SN, et al. Serum levels of hepatitis B surface antigen and DNA can predict inactive carriers with low risk of disease progression. Hepatology 2016;64:381-389.


114. Park H, Lee JM, Seo JH, Kim HS, Ahn SH, Kim DY, et al. Predictive value of HBsAg quantification for determining the clinical course of genotype C HBeAg-negative carriers. Liver Int 2012;32:796-802.


115. Tseng TC, Liu CJ, Yang HC, Su TH, Wang CC, Chen CL, et al. High levels of hepatitis B surface antigen increase risk of hepatocellular carcinoma in patients with low HBV load. Gastroenterology 2012;142:1140-1149 e3; quiz e13-e14.


116. Sonneveld MJ, Hansen BE, Piratvisuth T, Jia JD, Zeuzem S, Gane E, et al. Response-guided peginterferon therapy in hepatitis B e antigen-positive chronic hepatitis B using serum hepatitis B surface antigen levels. Hepatology 2013;58:872-880.


117. Chang ML, Liaw YF, Hadziyannis SJ. Systematic review: cessation of long-term nucleos(t)ide analogue therapy in patients with hepatitis B e antigen-negative chronic hepatitis B. Aliment Pharmacol Ther 2015;42:243-257.


119. Lai M, Hyatt BJ, Nasser I, Curry M, Afdhal NH. The clinical significance of persistently normal ALT in chronic hepatitis B infection. J Hepatol 2007;47:760-767.


122. Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003;38:518-526.


124. Xiao G, Yang J, Yan L. Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: a systemic review and meta-analysis. Hepatology 2015;61:292-302.


125. Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology 2007;46:32-36.


126. Kim BK, Kim DY, Park JY, Ahn SH, Chon CY, Kim JK, et al. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int 2010;30:546-553.


129. Lucidarme D, Foucher J, Le Bail B, Vergniol J, Castera L, Duburque C, et al. Factors of accuracy of transient elastography (fibroscan) for the diagnosis of liver fibrosis in chronic hepatitis C. Hepatology 2009;49:1083-1089.


130. Myers RP, Crotty P, Pomier-Layrargues G, Ma M, Urbanski SJ, Elkashab M. Prevalence, risk factors and causes of discordance in fibrosis staging by transient elastography and liver biopsy. Liver Int 2010;30:1471-1480.


131. Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol 2008;48:835-847.


132. Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol 2003;29:1705-1713.


133. Castera L, Pinzani M. Biopsy and non-invasive methods for the diagnosis of liver fibrosis: does it take two to tango? Gut 2010;59:861-866.


134. Castera L. Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology 2012;142:1293-1302 e4.


137. Chang W, Lee JM, Yoon JH, Han JK, Choi BI, Yoon JH, et al. Liver fibrosis staging with MR elastography: comparison of diagnostic performance between patients with chronic hepatitis B and those with other etiologic causes. Radiology 2016;280:88-97.


138. Morisaka H, Motosugi U, Ichikawa S, Nakazawa T, Kondo T, Funayama S, et al. Magnetic resonance elastography is as accurate as liver biopsy for liver fibrosis staging. J Magn Reson Imaging 2018;47:1268-1275.


140. Ichikawa S, Motosugi U, Morisaka H, Sano K, Ichikawa T, Tatsumi A, et al. Comparison of the diagnostic accuracies of magnetic resonance elastography and transient elastography for hepatic fibrosis. Magn Reson Imaging 2015;33:26-30.


144. Han KH, Kim DY, Park JY, Ahn SH, Kim J, Kim SU, et al. Survival of hepatocellular carcinoma patients may be improved in surveillance interval not more than 6 months compared with more than 6 months: a 15-year prospective study. J Clin Gastroenterol 2013;47:538-544.


145. Kim HY, Nam JY, Lee JH, Lee HA, Chang Y, Lee HY, et al. Intensity of surveillance for hepatocellular carcinoma determines survival in patients at risk in a hepatitis B-endemic area. Aliment Pharmacol Ther 2018;47:1490-1501.


146. Kim WR, Loomba R, Berg T, Aguilar Schall RE, Yee LJ, Dinh PV, et al. Impact of long-term tenofovir disoproxil fumarate on incidence of hepatocellular carcinoma in patients with chronic hepatitis B. Cancer 2015;121:3631-3638.


147. Liaw YF, Sheen IS, Lee CM, Akarca US, Papatheodoridis GV, SuetHing Wong F, et al. Tenofovir disoproxil fumarate (TDF), emtricitabine/TDF, and entecavir in patients with decompensated chronic hepatitis B liver disease. Hepatology 2011;53:62-72.


148. Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004;351:1521-1531.


149. Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label followup study. Lancet 2013;381:468-475.


150. Matsumoto A, Tanaka E, Rokuhara A, Kiyosawa K, Kumada H, Omata M, et al. Efficacy of lamivudine for preventing hepatocellular carcinoma in chronic hepatitis B: a multicenter retrospective study of 2795 patients. Hepatol Res 2005;32:173-184.


151. Shim JH, Lee HC, Kim KM, Lim YS, Chung YH, Lee YS, et al. Efficacy of entecavir in treatment-naïve patients with hepatitis B virusrelated decompensated cirrhosis. J Hepatol 2010;52:176-182.


153. Calvaruso V, Craxì A. Fibrosis in chronic viral hepatitis. Best Pract Res Clin Gastroenterol 2011;25:219-230.


155. Lok AS, McMahon BJ, Brown RS Jr, Wong JB, Ahmed AT, Farah W, et al. Antiviral therapy for chronic hepatitis B viral infection in adults: a systematic review and meta-analysis. Hepatology 2016;63:284-306.


156. Tang LSY, Covert E, Wilson E, Kottilil S. Chronic hepatitis B infection: a review. JAMA 2018;319:1802-1813.


157. Andreani T, Serfaty L, Mohand D, Dernaika S, Wendum D, Chazouill├©res O, et al. Chronic hepatitis B virus carriers in the immunotolerant phase of infection: histologic findings and outcome. Clin Gastroenterol Hepatol 2007;5:636-641.


159. Kim GA, Lim YS, Han S, Choi J, Shim JH, Kim KM, et al. High risk of hepatocellular carcinoma and death in patients with immunetolerant-phase chronic hepatitis B. Gut 2018;67:945-952.


160. Kim MN, Kim SU, Kim BK, Park JY, Kim DY, Ahn SH, et al. Increased risk of hepatocellular carcinoma in chronic hepatitis B patients with transient elastography-defined subclinical cirrhosis. Hepatology 2015;61:1851-1859.


162. Park JY, Park YN, Kim DY, Paik YH, Lee KS, Moon BS, et al. High prevalence of significant histology in asymptomatic chronic hepatitis B patients with genotype C and high serum HBV DNA levels. J Viral Hepat 2008;15:615-621.


163. Chan HL, Chan CK, Hui AJ, Chan S, Poordad F, Chang TT, et al. Effects of tenofovir disoproxil fumarate in hepatitis B e antigenpositive patients with normal levels of alanine aminotransferase and high levels of hepatitis B virus DNA. Gastroenterology 2014;146:1240-1248.


164. Wong VW, Hui AJ, Wong GL, Chan RS, Chim AM, Lo AO, et al. Four-year outcomes after cessation of tenofovir in immune-tolerant chronic hepatitis B patients. J Clin Gastroenterol 2018;52:347-352.


166. Cho JY, Paik YH, Sohn W, Cho HC, Gwak GY, Choi MS, et al. Patients with chronic hepatitis B treated with oral antiviral therapy retain a higher risk for HCC compared with patients with inactive stage disease. Gut 2014;63:1943-1950.


167. Choi J, Han S, Kim N, Lim YS. Increasing burden of liver cancer despite extensive use of antiviral agents in a hepatitis B virusendemic population. Hepatology 2017;66:1454-1463.


168. Yuen MF, Tanaka Y, Fong DY, Fung J, Wong DK, Yuen JC, et al. Independent risk factors and predictive score for the development of hepatocellular carcinoma in chronic hepatitis B. J Hepatol 2009;50:80-88.


170. Lee JK, Shim JH, Lee HC, Lee SH, Kim KM, Lim YS, et al. Estimation of the healthy upper limits for serum alanine aminotransferase in Asian populations with normal liver histology. Hepatology 2010;51:1577-1583.


172. Chao DT, Lim JK, Ayoub WS, Nguyen LH, Nguyen MH. Systematic review with meta-analysis: the proportion of chronic hepatitis B patients with normal alanine transaminase Ōēż 40 IU/L and significant hepatic fibrosis. Aliment Pharmacol Ther 2014;39:349-358.


173. Chen CF, Lee WC, Yang HI, Chang HC, Jen CL, Iloeje UH, et al. Changes in serum levels of HBV DNA and alanine aminotransferase determine risk for hepatocellular carcinoma. Gastroenterology 2011;141:1240-1248 1248.e1-e2.


175. Park HN, Sinn DH, Gwak GY, Kim JE, Rhee SY, Eo SJ, et al. Upper normal threshold of serum alanine aminotransferase in identifying individuals at risk for chronic liver disease. Liver Int 2012;32:937-944.


176. Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med 2006;354:1001-1010.


177. Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med 2003;348:800-807.


178. Lai CL, Gane E, Liaw YF, Hsu CW, Thongsawat S, Wang Y, et al. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med 2007;357:2576-2588.


179. Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med 2006;354:1011-1020.


180. Marcellin P, Chang TT, Lim SG, Tong MJ, Sievert W, Shiffman ML, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigenpositive chronic hepatitis B. N Engl J Med 2003;348:808-816.


181. Marcellin P, Heathcote EJ, Buti M, Gane E, de Man RA, Krastev Z, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med 2008;359:2442-2455.


182. Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD; American Association for the Study of Liver Diseases. Liver biopsy. Hepatology 2009;49:1017-1044.


183. European Association for Study of Liver; Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol 2015;63:237-264.


186. Kim HS, Kim HJ, Shin WG, Kim KH, Lee JH, Kim HY, et al. Predictive factors for early HBeAg seroconversion in acute exacerbation of patients with HBeAg-positive chronic hepatitis B. Gastroenterology 2009;136:505-512.


187. Huang KW, Tam KW, Luo JC, Kuan YC. Efficacy and safety of lamivudine versus entecavir for treating chronic hepatitis B virus-related acute exacerbation and acute-on-chronic liver failure: a systematic review and meta-analysis. J Clin Gastroenterol 2017;51:539-547.


189. Chen CH, Lee CM, Lu SN, Wang JH, Tung HD, Hung CH, et al. Comparison of clinical outcome between patients continuing and discontinuing lamivudine therapy after biochemical breakthrough of YMDD mutants. J Hepatol 2004;41:454-461.


192. Garg H, Sarin SK, Kumar M, Garg V, Sharma BC, Kumar A. Tenofovir improves the outcome in patients with spontaneous reactivation of hepatitis B presenting as acute-on-chronic liver failure. Hepatology 2011;53:774-780.


194. Wong VW, Wong GL, Yiu KK, Chim AM, Chu SH, Chan HY, et al. Entecavir treatment in patients with severe acute exacerbation of chronic hepatitis B. J Hepatol 2011;54:236-242.


196. Park JG, Lee YR, Park SY, Lee HJ, Tak WY, Kweon YO, et al. Tenofovir, entecavir, and lamivudine in patients with severe acute exacerbation and hepatic decompensation of chronic hepatitis B. Dig Liver Dis 2018;50:163-167.


197. Chen CH, Lin CL, Hu TH, Hung CH, Tseng PL, Wang JH, et al. Entecavir vs. lamivudine in chronic hepatitis B patients with severe acute exacerbation and hepatic decompensation. J Hepatol 2014;60:1127-1134.


198. Yasui S, Fujiwara K, Nakamura M, Miyamura T, Yonemitsu Y, Mikata R, et al. Virological efficacy of combination therapy with corticosteroid and nucleoside analogue for severe acute exacerbation of chronic hepatitis B. J Viral Hepat 2015;22:94-102.


201. Paik N, Sinn DH, Lee JH, Oh IS, Kim JH, Kang W, et al. Non-invasive tests for liver disease severity and the hepatocellular carcinoma risk in chronic hepatitis B patients with low-level viremia. Liver Int 2018;38:68-75.


202. de Niet A, Jansen L, Stelma F, Willemse SB, Kuiken SD, Weijer S, et al. Peg-interferon plus nucleotide analogue treatment versus no treatment in patients with chronic hepatitis B with a low viral load: a randomised controlled, open-label trial. Lancet Gastroenterol Hepatol 2017;2:576-584.


203. Chang TT, Liaw YF, Wu SS, Schiff E, Han KH, Lai CL, et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology 2010;52:886-893.


205. Kim JH, Sinn DH, Kang W, Gwak GY, Paik YH, Choi MS, et al. Lowlevel viremia and the increased risk of hepatocellular carcinoma in patients receiving entecavir treatment. Hepatology 2017;66:335-343.


206. Sinn DH, Lee J, Goo J, Kim K, Gwak GY, Paik YH, et al. Hepatocellular carcinoma risk in chronic hepatitis B virus-infected compensated cirrhosis patients with low viral load. Hepatology 2015;62:694-701.


207. Cho YY, Lee JH, Chang Y, Nam JY, Cho H, Lee DH, et al. Comparison of overall survival between antiviral-induced viral suppression and inactive phase chronic hepatitis B patients. J Viral Hepat 2018;25:1161-1171.


209. Jang JW, Choi JY, Kim YS, Woo HY, Choi SK, Lee CH, et al. Longterm effect of antiviral therapy on disease course after decompensation in patients with hepatitis B virus-related cirrhosis. Hepatology 2015;61:1809-1820.


210. Fontana RJ, Hann HW, Perrillo RP, Vierling JM, Wright T, Rakela J, et al. Determinants of early mortality in patients with decompensated chronic hepatitis B treated with antiviral therapy. Gastroenterology 2002;123:719-727.


214. Yim SY, Um SH, Jung JY, Seo YS, Yim HJ, Ryu HS, et al. Role of hepatitis B surface antigen (HBsAg) in identifying true inactive HBsAg carriers infected with genotype C hepatitis B virus. J Clin Gastroenterol 2014;48:166-171.


216. Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, Cooksley G, et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med 2005;352:2682-2695.


218. Qiu K, Liu B, Li SY, Li H, Chen ZW, Luo AR, et al. Systematic review with meta-analysis: combination treatment of regimens based on pegylated interferon for chronic hepatitis B focusing on hepatitis B surface antigen clearance. Aliment Pharmacol Ther 2018;47:1340-1348.


219. Vigan├▓ M, Grossi G, Loglio A, Lampertico P. Treatment of hepatitis B: is there still a role for interferon? Liver Int 2018;38 Suppl 1:79-83.


220. Janssen HL, van Zonneveld M, Senturk H, Zeuzem S, Akarca US, Cakaloglu Y, et al. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet 2005;365:123-129.


224. Agarwal K, Brunetto M, Seto WK, Lim YS, Fung S, Marcellin P, et al. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection. J Hepatol 2018;68:672-681.


225. Chan HL, Fung S, Seto WK, Chuang WL, Chen CY, Kim HJ, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAg-positive chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol 2016;1:185-195.


226. Buti M, Gane E, Seto WK, Chan HL, Chuang WL, Stepanova T, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol 2016;1:196-206.


227. Seto WK, Asahina Y, Brown TT, Peng CY, Stanciu C, Abdurakhmanov D, et al. Improved bone safety of tenofovir alafenamide compared to tenofovir disoproxil fumarate over 2 years in patients with chronic HBV infection. Clin Gastroenterol Hepatol 2018 Jun 20;pii: S1542-3565(18)30633-5. doi: 10.1016/j.cgh.2018.06.023.

229. Yuen MF, Lee SH, Kang HM, Kim CR, Kim J, Ngai V, et al. Pharmacokinetics of LB80331 and LB80317 following oral administration of LB80380, a new antiviral agent for chronic hepatitis B (CHB), in healthy adult subjects, CHB patients, and mice. Antimicrob Agents Chemother 2009;53:1779-1785.



230. Ahn SH, Kim W, Jung YK, Yang JM, Jang JY, Kweon YO, et al. Safety and efficacy of besifovir in treatment-naïve chronic hepatitis B virus infection: a randomized, double-blind, double dummy, phase 3 study [Abstract]. J Hepatol 2017;66:S88-S89.

232. Hou J, Zhao W, Lee C, Hann HW, Peng CY, Tanwandee T, et al. Prospective, randomized assessment of HBV-associated and other clinical outcome events during long-term therapy with entecavir or other HBV nucleos(t)ide analogues in patients with chronic HBV infection [Abstract]. Hepatology 2017;66(S1):12A-13A.
233. Liu Y, Corsa AC, Buti M, Cathcart AL, Flaherty JF, Miller MD, et al. No detectable resistance to tenofovir disoproxil fumarate in HBeAg+ and HBeAg- patients with chronic hepatitis B after 8 years of treatment. J Viral Hepat 2017;24:68-74.


234. Konerman MA, Lok AS. Interferon treatment for hepatitis B. Clin Liver Dis 2016;20:645-665.


235. Cooksley WG, Piratvisuth T, Lee SD, Mahachai V, Chao YC, Tanwandee T, et al. Peginterferon alpha-2a (40 kDa): an advance in the treatment of hepatitis B e antigen-positive chronic hepatitis B. J Viral Hepat 2003;10:298-305.


236. Chan HL, Leung NW, Hui AY, Wong VW, Liew CT, Chim AM, et al. A randomized, controlled trial of combination therapy for chronic hepatitis B: comparing pegylated interferon-alpha2b and lamivudine with lamivudine alone. Ann Intern Med 2005;142:240-250.


237. Buster EH, Flink HJ, Cakaloglu Y, Simon K, Trojan J, Tabak F, et al. Sustained HBeAg and HBsAg loss after long-term follow-up of HBeAg-positive patients treated with peginterferon alpha-2b. Gastroenterology 2008;135:459-467.


238. Liaw YF, Jia JD, Chan HL, Han KH, Tanwandee T, Chuang WL, et al. Shorter durations and lower doses of peginterferon alfa-2a are associated with inferior hepatitis B e antigen seroconversion rates in hepatitis B virus genotypes B or C. Hepatology 2011;54:1591-1599.


241. Song G, Yang R, Rao H, Feng B, Ma H, Jin Q, et al. Serum HBV core-related antigen is a good predictor for spontaneous HBeAg seroconversion in chronic hepatitis B patients. J Med Virol 2017;89:463-468.


243. You H, Ma H, Liu T, Cong M, Wang P, Ou X, et al. Different models of HBeAg seroconversion predicated by on-treatment ALT and HBV DNA profiles. J Viral Hepat 2009;16:876-882.


244. Zeuzem S, Gane E, Liaw YF, Lim SG, DiBisceglie A, Buti M, et al. Baseline characteristics and early on-treatment response predict the outcomes of 2 years of telbivudine treatment of chronic hepatitis B. J Hepatol 2009;51:11-20.


246. Chan HL, Wong VW, Tse CH, Chim AM, Chan HY, Wong GL, et al. Early virological suppression is associated with good maintained response to adefovir dipivoxil in lamivudine resistant chronic hepatitis B. Aliment Pharmacol Ther 2007;25:891-898.


247. Yuen MF, Fong DY, Wong DK, Yuen JC, Fung J, Lai CL. Hepatitis B virus DNA levels at week 4 of lamivudine treatment predict the 5-year ideal response. Hepatology 2007;46:1695-1703.


248. Gish RG, Chang TT, Lai CL, de Man R, Gadano A, Poordad F, et al. Loss of HBsAg antigen during treatment with entecavir or lamivudine in nucleoside-naïve HBeAg-positive patients with chronic hepatitis B. J Viral Hepat 2010;17:16-22.


250. Fung S, Gordon SC, Krastev Z, Horban A, Petersen J, Sperl J, et al. Tenofovir disoproxil fumarate in Asian or Pacific Islander chronic hepatitis B patients with high viral load (Ōēź 9 log10 copies/ml). Liver Int 2015;35:422-428.


251. Buster EH, Hansen BE, Lau GK, Piratvisuth T, Zeuzem S, Steyerberg EW, et al. Factors that predict response of patients with hepatitis B e antigen-positive chronic hepatitis B to peginterferon-alfa. Gastroenterology 2009;137:2002-2009.


252. Kong LN, Qin B, Ma Q, Li L, Yao Y. Relationship between hepatitis B virus genotype B and C and response to interferon therapy in HBeAg positive chronic hepatitis B patients: a meta-analysis. J Gastroenterol Hepatol 2014;29:1387-1395.


253. Chan HLY, Messinger D, Papatheodoridis GV, Cornberg M, Xie Q, Piratvisuth T, et al. A baseline tool for predicting response to peginterferon alfa-2a in HBeAg-positive patients with chronic hepatitis B. Aliment Pharmacol Ther 2018;48:547-555.


254. Goulis I, Karatapanis S, Akriviadis E, Deutsch M, Dalekos GN, Raptopoulou-Gigi M, et al. On-treatment prediction of sustained response to peginterferon alfa-2a for HBeAg-negative chronic hepatitis B patients. Liver Int 2015;35:1540-1548.


255. Moucari R, Mackiewicz V, Lada O, Ripault MP, Castelnau C, Martinot-Peignoux M, et al. Early serum HBsAg drop: a strong predictor of sustained virological response to pegylated interferon alfa-2a in HBeAg-negative patients. Hepatology 2009;49:1151-1157.


257. Locarnini S, Mason WS. Cellular and virological mechanisms of HBV drug resistance. J Hepatol 2006;44:422-431.


258. Yuan HJ, Yuen MF, Ka-Ho Wong D, Sablon E, Lai CL. The relationship between HBV-DNA levels and cirrhosis-related complications in Chinese with chronic hepatitis B. J Viral Hepat 2005;12:373-379.


259. Jung YK, Kim JH, Lee YS, Lee HJ, Yoon E, Jung ES, et al. Change in serum hepatitis B surface antigen level and its clinical significance in treatment-naïve, hepatitis B e antigen-positive patients receiving entecavir. J Clin Gastroenterol 2010;44:653-657.


261. Reijnders JG, Rijckborst V, Sonneveld MJ, Scherbeijn SM, Boucher CA, Hansen BE, et al. Kinetics of hepatitis B surface antigen differ between treatment with peginterferon and entecavir. J Hepatol 2011;54:449-454.


262. Gramenzi A, Loggi E, Micco L, Cursaro C, Fiorino S, Galli S, et al. Serum hepatitis B surface antigen monitoring in long-term lamivudine-treated hepatitis B virus patients. J Viral Hepat 2011;18:e468-e474.


263. Kwon JH, Jang JW, Lee S, Lee J, Chung KW, Lee YS, et al. Pretreatment HBeAg level and an early decrease in HBeAg level predict virologic response to entecavir treatment for HBeAg-positive chronic hepatitis B. J Viral Hepat 2012;19:e41-e47.


264. Zoulim F, Carosi G, Greenbloom S, Mazur W, Nguyen T, Jeffers L, et al. Quantification of HBsAg in nucleos(t)ide-naïve patients treated for chronic hepatitis B with entecavir with or without tenofovir in the BE-LOW study. J Hepatol 2015;62:56-63.


265. Seto WK, Wong DK, Fung J, Huang FY, Lai CL, Yuen MF. Reduction of hepatitis B surface antigen levels and hepatitis B surface antigen seroclearance in chronic hepatitis B patients receiving 10 years of nucleoside analogue therapy. Hepatology 2013;58:923-931.


266. Chen CH, Hung CH, Hu TH, Wang JH, Lu SN, Su PF, et al. Association between level of hepatitis B surface antigen and relapse after entecavir therapy for chronic hepatitis B virus infection. Clin Gastroenterol Hepatol 2015;13:1984-1992 e1.


267. Chen CH, Lu SN, Hung CH, Wang JH, Hu TH, Changchien CS, et al. The role of hepatitis B surface antigen quantification in predicting HBsAg loss and HBV relapse after discontinuation of lamivudine treatment. J Hepatol 2014;61:515-522.


268. Hsu YC, Mo LR, Chang CY, Wu MS, Kao JH, Wang WL, et al. Association between serum level of hepatitis B surface antigen at end of entecavir therapy and risk of relapse in E antigen-negative patients. Clin Gastroenterol Hepatol 2016;14:1490-1498 e3.


272. Lee JM, Park JY, Kim DY, Nguyen T, Hong SP, Kim SO, et al. Longterm adefovir dipivoxil monotherapy for up to 5 years in lamivudine-resistant chronic hepatitis B. Antivir Ther 2010;15:235-241.


274. Samarkos M, Theofanis V, Eliadi I, Vlachogiannakos J, Polyzos A. Tenofovir-associated Fanconi syndrome in a patient with chronic hepatitis B. J Gastrointestin Liver Dis 2014;23:342.
275. Fleischer RD, Lok AS. Myopathy and neuropathy associated with nucleos(t)ide analog therapy for hepatitis B. J Hepatol 2009;51:787-791.


276. Kim BK, Oh J, Kwon SY, Choe WH, Ko SY, Rhee KH, et al. Clevudine myopathy in patients with chronic hepatitis B. J Hepatol 2009;51:829-834.


277. Tak WY, Park SY, Cho CM, Jung MK, Jeon SW, Kweon YO, et al. Clinical, biochemical, and pathological characteristics of clevudineassociated myopathy. J Hepatol 2010;53:261-266.


278. Brunetto MR, Moriconi F, Bonino F, Lau GK, Farci P, Yurdaydin C, et al. Hepatitis B virus surface antigen levels: a guide to sustained response to peginterferon alfa-2a in HBeAg-negative chronic hepatitis B. Hepatology 2009;49:1141-1150.


280. Chen X, Chen X, Chen W, Ma X, Huang J, Chen R. Extended peginterferon alfa-2a (Pegasys) therapy in Chinese patients with HBeAgnegative chronic hepatitis B. J Med Virol 2014;86:1705-1713.


281. Lampertico P, Vigan├▓ M, Di Costanzo GG, Sagnelli E, Fasano M, Di Marco V, et al. Randomised study comparing 48 and 96 weeks peginterferon ╬▒-2a therapy in genotype D HBeAg-negative chronic hepatitis B. Gut 2013;62:290-298.


282. Kang HS, Um SH, Seo YS, An H, Lee KG, Hyun JJ, et al. Healthy range for serum ALT and the clinical significance of ŌĆ£unhealthyŌĆØ normal ALT levels in the Korean population. J Gastroenterol Hepatol 2011;26:292-299.


283. Fattovich G, Olivari N, Pasino M, DŌĆÖOnofrio M, Martone E, Donato F. Long-term outcome of chronic hepatitis B in Caucasian patients: mortality after 25 years. Gut 2008;57:84-90.


284. Fattovich G, Pantalena M, Zagni I, Realdi G, Schalm SW, Christensen E, et al. Effect of hepatitis B and C virus infections on the natural history of compensated cirrhosis: a cohort study of 297 patients. Am J Gastroenterol 2002;97:2886-2895.


285. Mommeja-Marin H, Mondou E, Blum MR, Rousseau F. Serum HBV DNA as a marker of efficacy during therapy for chronic HBV infection: analysis and review of the literature. Hepatology 2003;37:1309-1319.


286. Hadziyannis SJ, Sevastianos V, Rapti I, Vassilopoulos D, Hadziyannis E. Sustained responses and loss of HBsAg in HBeAg-negative patients with chronic hepatitis B who stop long-term treatment with adefovir. Gastroenterology 2012;143:629-636 e1.


287. Papatheodoridis G, Vlachogiannakos I, Cholongitas E, Wursthorn K, Thomadakis C, Touloumi G, et al. Discontinuation of oral antivirals in chronic hepatitis B: a systematic review. Hepatology 2016;63:1481-1492.


288. Berg T, Simon KG, Mauss S, Schott E, Heyne R, Klass DM, et al. Long-term response after stopping tenofovir disoproxil fumarate in non-cirrhotic HBeAg-negative patients - FINITE study. J Hepatol 2017;67:918-924.


290. Jeng WJ, Sheen IS, Chen YC, Hsu CW, Chien RN, Chu CM, et al. Off-therapy durability of response to entecavir therapy in hepatitis B e antigen-negative chronic hepatitis B patients. Hepatology 2013;58:1888-1896.


292. Seto WK, Hui AJ, Wong VW, Wong GL, Liu KS, Lai CL, et al. Treatment cessation of entecavir in Asian patients with hepatitis B e antigen negative chronic hepatitis B: a multicentre prospective study. Gut 2015;64:667-672.


293. Fattovich G, Rugge M, Brollo L, Pontisso P, Noventa F, Guido M, et al. Clinical, virologic and histologic outcome following seroconversion from HBeAg to anti-HBe in chronic hepatitis type B. Hepatology 1986;6:167-172.


294. Yang HI, Lu SN, Liaw YF, You SL, Sun CA, Wang LY, et al. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med 2002;347:168-174.


295. Niederau C, Heintges T, Lange S, Goldmann G, Niederau CM, Mohr L, et al. Long-term follow-up of HBeAg-positive patients treated with interferon alfa for chronic hepatitis B. N Engl J Med 1996;334:1422-1427.


296. Reijnders JG, Perquin MJ, Zhang N, Hansen BE, Janssen HL. Nucleos(t)ide analogues only induce temporary hepatitis B e antigen seroconversion in most patients with chronic hepatitis B. Gastroenterology 2010;139:491-498.


297. Song BC, Suh DJ, Lee HC, Chung YH, Lee YS. Hepatitis B e antigen seroconversion after lamivudine therapy is not durable in patients with chronic hepatitis B in Korea. Hepatology 2000;32(4 Pt 1):803-806.


298. Byun KS, Kwon OS, Kim JH, Yim HJ, Chang YJ, Kim JY, et al. Factors related to post-treatment relapse in chronic hepatitis B patients who lost HBeAg after lamivudine therapy. J Gastroenterol Hepatol 2005;20:1838-1842.


299. Lee HW, Lee HJ, Hwang JS, Sohn JH, Jang JY, Han KJ, et al. Lamivudine maintenance beyond one year after HBeAg seroconversion is a major factor for sustained virologic response in HBeAg-positive chronic hepatitis B. Hepatology 2010;51:415-421.


300. Chan HL, Wong VW, Tse AM, Tse CH, Chim AM, Chan HY, et al. Serum hepatitis B surface antigen quantitation can reflect hepatitis B virus in the liver and predict treatment response. Clin Gastroenterol Hepatol 2007;5:1462-1468.


301. Kim JH, Lee YS, Lee HJ, Yoon E, Jung YK, Jong ES, et al. HBsAg seroclearance in chronic hepatitis B: implications for hepatocellular carcinoma. J Clin Gastroenterol 2011;45:64-68.


302. Yip TC, Wong GL, Wong VW, Tse YK, Lui GC, Lam KL, et al. Durability of hepatitis B surface antigen seroclearance in untreated and nucleos(t)ide analogue-treated patients. J Hepatol 2017 Oct 6;pii: S0168-8278(17)32332-2. doi: 10.1016/j.jhep.2017.09.018.

303. Chevaliez S, H├®zode C, Bahrami S, Grare M, Pawlotsky JM. Longterm hepatitis B surface antigen (HBsAg) kinetics during nucleoside/nucleotide analogue therapy: finite treatment duration unlikely. J Hepatol 2013;58:676-683.


304. Seto WK, Liu K, Wong DK, Fung J, Huang FY, Hung IF, et al. Patterns of hepatitis B surface antigen decline and HBV DNA suppression in Asian treatment-experienced chronic hepatitis B patients after three years of tenofovir treatment. J Hepatol 2013;59:709-716.


305. Fung J, Cheung KS, Wong DK, Mak LY, To WP, Seto WK, et al. Long term outcomes and predictive scores for hepatocellular carcinoma and hepatitis B surface antigen seroclearance after hepatitis B eantigen seroclearance. Hepatology 2018;68:462-472.


306. Kim GA, Lee HC, Kim MJ, Ha Y, Park EJ, An J, et al. Incidence of hepatocellular carcinoma after HBsAg seroclearance in chronic hepatitis B patients: a need for surveillance. J Hepatol 2015;62:1092-1099.


307. Nafa S, Ahmed S, Tavan D, Pichoud C, Berby F, Stuyver L, et al. Early detection of viral resistance by determination of hepatitis B virus polymerase mutations in patients treated by lamivudine for chronic hepatitis B. Hepatology 2000;32:1078-1088.


308. Zoulim F, Durantel D, Deny P. Management and prevention of drug resistance in chronic hepatitis B. Liver Int 2009;29 Suppl 1:108-115.


310. Melegari M, Scaglioni PP, Wands JR. Hepatitis B virus mutants associated with 3TC and famciclovir administration are replication defective. Hepatology 1998;27:628-633.


313. Lok AS, Lai CL, Leung N, Yao GB, Cui ZY, Schiff ER, et al. Longterm safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology 2003;125:1714-1722.


314. Lok AS, Zoulim F, Locarnini S, Bartholomeusz A, Ghany MG, Pawlotsky JM, et al. Antiviral drug-resistant HBV: standardization of nomenclature and assays and recommendations for management. Hepatology 2007;46:254-265.


315. Gish RG, Trinh H, Leung N, Chan FK, Fried MW, Wright TL, et al. Safety and antiviral activity of emtricitabine (FTC) for the treatment of chronic hepatitis B infection: a two-year study. J Hepatol 2005;43:60-66.


317. Liaw YF, Gane E, Leung N, Zeuzem S, Wang Y, Lai CL, et al. 2-Year GLOBE trial results: telbivudine is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology 2009;136:486-495.


318. Koh KH, Kang CJ, Kim DH, Choi YW, Kim MJ, Cheong JY, et al. Development of clevudine resistance after switching from lamivudine in a patient with chronic hepatitis B. Korean J Gastroenterol 2008;52:325-328.

319. Yoon EL, Yim HJ, Lee HJ, Lee YS, Kim JH, Jung ES, et al. Comparison of clevudine and entecavir for treatment-naive patients with chronic hepatitis B virus infection: two-year follow-up data. J Clin Gastroenterol 2011;45:893-899.


323. Tenney DJ, Rose RE, Baldick CJ, Pokornowski KA, Eggers BJ, Fang J, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naïve patients is rare through 5 years of therapy. Hepatology 2009;49:1503-1514.


325. Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology 2006;131:1743-1751.


326. Villeneuve JP, Durantel D, Durantel S, Westland C, Xiong S, Brosgart CL, et al. Selection of a hepatitis B virus strain resistant to adefovir in a liver transplantation patient. J Hepatol 2003;39:1085-1089.


327. Villet S, Pichoud C, Billioud G, Barraud L, Durantel S, Tr├®po C, et al. Impact of hepatitis B virus rtA181V/T mutants on hepatitis B treatment failure. J Hepatol 2008;48:747-755.


328. Lampertico P, Vigan├▓ M, Manenti E, Iavarone M, Sablon E, Colombo M. Low resistance to adefovir combined with lamivudine: a 3-year study of 145 lamivudine-resistant hepatitis B patients. Gastroenterology 2007;133:1445-1451.


331. Amini-Bavil-Olyaee S, Herbers U, Sheldon J, Luedde T, Trautwein C, Tacke F. The rtA194T polymerase mutation impacts viral replication and susceptibility to tenofovir in hepatitis B e antigen-positive and hepatitis B e antigen-negative hepatitis B virus strains. Hepatology 2009;49:1158-1165.


333. Park ES, Lee AR, Kim DH, Lee JH, Yoo JJ, Ahn SH, et al. Identification of a quadruple mutation that confers tenofovir resistance in chronic hepatitis B patients. J Hepatol 2019;70:1093-1102.


334. Ahn SH, Kim W, Jung YK, Yang JM, Jang JY, Kweon YO, et al. Efficacy and safety of besifovir dipivoxil maleate compared with tenofovir disoproxil fumarate in treatment of chronic hepatitis B virus infection. Clin Gastroenterol Hepatol 2018 Nov 15;pii: S1542- 3565(18)31244-8. doi: 10.1016/j.cgh.2018.11.001.

336. Yim HJ, Hussain M, Liu Y, Wong SN, Fung SK, Lok AS. Evolution of multi-drug resistant hepatitis B virus during sequential therapy. Hepatology 2006;44:703-712.


339. Yim HJ. Management of antiviral-resistant chronic hepatitis B virus infection. Korean J Gastroenterol 2008;51:346-359.

340. Fung S, Kwan P, Fabri M, Horban A, Pelemis M, Hann HW, et al. Randomized comparison of tenofovir disoproxil fumarate vs emtricitabine and tenofovir disoproxil fumarate in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology 2014;146:980-988.


341. Lim YS, Byun KS, Yoo BC, Kwon SY, Kim YJ, An J, et al. Tenofovir monotherapy versus tenofovir and entecavir combination therapy in patients with entecavir-resistant chronic hepatitis B with multiple drug failure: results of a randomised trial. Gut 2016;65:852-860.


342. Lee S, Ahn SH, Jung KS, Kim DY, Kim BK, Kim SU, et al. Tenofovir versus tenofovir plus entecavir for chronic hepatitis B with lamivudine resistance and entecavir resistance. J Viral Hepat 2017;24:141-147.


343. Berg T, Zoulim F, Moeller B, Trinh H, Marcellin P, Chan S, et al. Long-term efficacy and safety of emtricitabine plus tenofovir DF vs. tenofovir DF monotherapy in adefovir-experienced chronic hepatitis B patients. J Hepatol 2014;60:715-722.


344. Lim YS, Yoo BC, Byun KS, Kwon SY, Kim YJ, An J, et al. Tenofovir monotherapy versus tenofovir and entecavir combination therapy in adefovir-resistant chronic hepatitis B patients with multiple drug failure: results of a randomised trial. Gut 2016;65:1042-1051.


345. Lim YS, Lee YS, Gwak GY, Byun KS, Kim YJ, Choi J, et al. Monotherapy with tenofovir disoproxil fumarate for multiple drug-resistant chronic hepatitis B: 3-year trial. Hepatology 2017;66:772-783.


348. Park JY, Kim CW, Bae SH, Jung KS, Kim HY, Yoon SK, et al. Entecavir plus tenofovir combination therapy in patients with multidrugresistant chronic hepatitis B: results of a multicentre, prospective study. Liver Int 2016;36:1108-1115.


349. Yim HJ, Suh SJ, Jung YK, Hwang SG, Park H, Seo YS, et al. Tenofovir-based combination therapy or monotherapy for multi-drug resistant chronic hepatitis B; real world data from multicenter cohort study [Abstract]. Hepatology 2017;66:12A.
350. Lee JH, Lee YB, Cho H, Cho YY, Cho EJ, Kim YJ, et al. Identification of a triple mutation that confers tenofovir resistance in chronic hepatitis B patients [Abstract]. Hepatology 2017;66:69A-70A.

351. Heo J, Park JY, Lee HJ, Tak WY, Um SH, Kim DY, et al. A 96-week randomized trial of switching to entecavir in chronic hepatitis B patients with a partial virological response to lamivudine. Antivir Ther 2012;17:1563-1570.


352. Yim HJ, Kim IH, Suh SJ, Jung YK, Kim JH, Seo YS, et al. Switching to tenofovir vs continuing entecavir for hepatitis B virus with partial virologic response to entecavir: a randomized controlled trial. J Viral Hepat 2018;25:1321-1330.


353. Chen J, Zhao SS, Liu XX, Huang ZB, Huang Y. Comparison of the efficacy of tenofovir versus tenofovir plus entecavir in the treatment of chronic hepatitis B in patients with poor efficacy of entecavir: a systematic review and meta-analysis. Clin Ther 2017;39:1870-1880.


355. Lee HW, Park JY, Lee JW, Yoon KT, Kim CW, Park H, et al. Longterm efficacy of tenofovir disoproxil fumarate monotherapy for multidrug-resistant chronic HBV infection. Clin Gastroenterol Hepatol 2018 Oct 26;pii: S1542-3565(18)31201-1. doi: 10.1016/j.cgh.2018.10.037.

356. Kim DY, Lee HW, Song JE, Kim BK, Kim SU, Kim DY, et al. Switching from tenofovir and nucleoside analogue therapy to tenofovir monotherapy in virologically suppressed chronic hepatitis B patients with antiviral resistance. J Med Virol 2018;90:497-502.


358. Ning Q, Han M, Sun Y, Jiang J, Tan D, Hou J, et al. Switching from entecavir to PegIFN alfa-2a in patients with HBeAg-positive chronic hepatitis B: a randomised open-label trial (OSST trial). J Hepatol 2014;61:777-784.


360. Xie Q, Zhou H, Bai X, Wu S, Chen JJ, Sheng J, et al. A randomized, open-label clinical study of combined pegylated interferon Alfa-2a (40KD) and entecavir treatment for hepatitis B ŌĆ£eŌĆØ antigen-positive chronic hepatitis B. Clin Infect Dis 2014;59:1714-1723.


362. Zhang W, Xie Q, Ning Q, Dou X, Chen X, Jia J, et al. The role of peginterferon in nucleos(t)ide-analogue-treated chronic hepatitis B patients: a review of published literature. J Viral Hepat 2017;24:618-623.


363. Bourli├©re M, Rabiega P, Ganne-Carrie N, Serfaty L, Marcellin P, Barthe Y, et al. Effect on HBs antigen clearance of addition of pegylated interferon alfa-2a to nucleos(t)ide analogue therapy versus nucleos(t)ide analogue therapy alone in patients with HBe antigennegative chronic hepatitis B and sustained undetectable plasma hepatitis B virus DNA: a randomised, controlled, open-label trial. Lancet Gastroenterol Hepatol 2017;2:177-188.


364. Lee JH, Chung S, Kim MS, Kim SW, Yoon JS, Chang Y, et al. Entecavir plus pegylated interferon alfa-2a and sequential HBV vaccination increases the chance of HBsAg-seroclearance: a results from randomized controlled E+VIP Trial [Abstract]. Clin Mol Hepatol 2018;24(Suppl 3):2.

365. Rijckborst V, Hansen BE, Ferenci P, Brunetto MR, Tabak F, Cakaloglu Y, et al. Validation of a stopping rule at week 12 using HBsAg and HBV DNA for HBeAg-negative patients treated with peginterferon alfa-2a. J Hepatol 2012;56:1006-1011.


366. Wu CY, Chen YJ, Ho HJ, Hsu YC, Kuo KN, Wu MS, et al. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. JAMA 2012;308:1906-1914.


367. Huang G, Li PP, Lau WY, Pan ZY, Zhao LH, Wang ZG, et al. Antiviral therapy reduces hepatocellular carcinoma recurrence in patients with low HBV-DNA levels: a randomized controlled trial. Ann Surg 2018;268:943-954.


368. Wong JS, Wong GL, Tsoi KK, Wong VW, Cheung SY, Chong CN, et al. Meta-analysis: the efficacy of anti-viral therapy in prevention of recurrence after curative treatment of chronic hepatitis B-related hepatocellular carcinoma. Aliment Pharmacol Ther 2011;33:1104-1112.


369. Cho H, Ahn H, Lee DH, Lee JH, Jung YJ, Chang Y, et al. Entecavir and tenofovir reduce hepatitis B virus-related hepatocellular carcinoma recurrence more effectively than other antivirals. J Viral Hepat 2018;25:707-717.


370. Chen LT, Chen MF, Li LA, Lee PH, Jeng LB, Lin DY, et al. Long-term results of a randomized, observation-controlled, phase III trial of adjuvant interferon Alfa-2b in hepatocellular carcinoma after curative resection. Ann Surg 2012;255:8-17.


372. Huang L, Li J, Yan J, Sun J, Zhang X, Wu M, et al. Antiviral therapy decreases viral reactivation in patients with hepatitis B virus-related hepatocellular carcinoma undergoing hepatectomy: a randomized controlled trial. J Viral Hepat 2013;20:336-342.


373. Dan JQ, Zhang YJ, Huang JT, Chen MS, Gao HJ, Peng ZW, et al. Hepatitis B virus reactivation after radiofrequency ablation or hepatic resection for HBV-related small hepatocellular carcinoma: a retrospective study. Eur J Surg Oncol 2013;39:865-872.


375. Lao XM, Luo G, Ye LT, Luo C, Shi M, Wang D, et al. Effects of antiviral therapy on hepatitis B virus reactivation and liver function after resection or chemoembolization for hepatocellular carcinoma. Liver Int 2013;33:595-604.


376. Lao XM, Wang D, Shi M, Liu G, Li S, Guo R, et al. Changes in hepatitis B virus DNA levels and liver function after transcatheter arterial chemoembolization of hepatocellular carcinoma. Hepatol Res 2011;41:553-563.


377. Firpi RJ, Nelson DR. Management of viral hepatitis in hematologic malignancies. Blood Rev 2008;22:117-126.


378. Jang JW, Choi JY, Bae SH, Yoon SK, Chang UI, Kim CW, et al. A randomized controlled study of preemptive lamivudine in patients receiving transarterial chemo-lipiodolization. Hepatology 2006;43:233-240.


379. Park JW, Park KW, Cho SH, Park HS, Lee WJ, Lee DH, et al. Risk of hepatitis B exacerbation is low after transcatheter arterial chemoembolization therapy for patients with HBV-related hepatocellular carcinoma: report of a prospective study. Am J Gastroenterol 2005;100:2194-2200.


381. Nagamatsu H, Itano S, Nagaoka S, Akiyoshi J, Matsugaki S, Kurogi J, et al. Prophylactic lamivudine administration prevents exacerbation of liver damage in HBe antigen positive patients with hepatocellular carcinoma undergoing transhepatic arterial infusion chemotherapy. Am J Gastroenterol 2004;99:2369-2375.


382. Tamori A, Nishiguchi S, Tanaka M, Kurooka H, Fujimoto S, Nakamura K, et al. Lamivudine therapy for hepatitis B virus reactivation in a patient receiving intra-arterial chemotherapy for advanced hepatocellular carcinoma. Hepatol Res 2003;26:77-80.


383. Kim JH, Park JW, Kim TH, Koh DW, Lee WJ, Kim CM. Hepatitis B virus reactivation after three-dimensional conformal radiotherapy in patients with hepatitis B virus-related hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2007;69:813-819.


384. Jang JW, Kwon JH, You CR, Kim JD, Woo HY, Bae SH, et al. Risk of HBV reactivation according to viral status and treatment intensity in patients with hepatocellular carcinoma. Antivir Ther 2011;16:969-977.


386. Suh SJ, Yim HJ, Seo JH, Lee YS, Hyun JJ, Jung YK, et al. The risk of hepatitis B virus reactivation is considerably high during sorafenib therapy in patients with advanced hepatocellular carcinoma. J Hepatol 2017;66:S449-S450.

388. Lampertico P, Chan HL, Janssen HL, Strasser SI, Schindler R, Berg T. Review article: long-term safety of nucleoside and nucleotide analogues in HBV-monoinfected patients. Aliment Pharmacol Ther 2016;44:16-34.


389. Gracey DM, Snelling P, McKenzie P, Strasser SI. Tenofovir-associated Fanconi syndrome in patients with chronic hepatitis B monoinfection. Antivir Ther 2013;18:945-948.


391. L├│pez-Alcorocho JM, Barril G, Ortiz-Movilla N, Traver JA, Bartolom├® J, Sanz P, et al. Prevalence of hepatitis B, hepatitis C, GB virus C/hepatitis G and TT viruses in predialysis and hemodialysis patients. J Med Virol 2001;63:103-107.


392. Gwak GY, Huh W, Lee DH, Min BH, Koh KC, Kim JJ, et al. Occult hepatitis B virus infection in chronic hemodialysis patients in Korea. Hepatogastroenterology 2008;55:1721-1724.

393. Minuk GY, Sun DF, Greenberg R, Zhang M, Hawkins K, Uhanova J, et al. Occult hepatitis B virus infection in a North American adult hemodialysis patient population. Hepatology 2004;40:1072-1077.


394. Burdick RA, Bragg-Gresham JL, Woods JD, Hedderwick SA, Kurokawa K, Combe C, et al. Patterns of hepatitis B prevalence and seroconversion in hemodialysis units from three continents: the DOPPS. Kidney Int 2003;63:2222-2229.


396. Finelli L, Miller JT, Tokars JI, Alter MJ, Arduino MJ. National surveillance of dialysis-associated diseases in the United States, 2002. Semin Dial 2005;18:52-61.


397. Hu TH, Huang PY, Chang KC, Tseng PL, Yen YH, Tsai MC. Chronic hepatitis B patient treated with Tenofovir (TDF) may experience increased risks of orthopedic disorders and renal deficits compared to those treated with Enetecavir (ETV) [Abstract]. Hepatology 2018;68(S1):264A.
398. Lai CL, Ahn SH, Lee KS, Um SH, Cho M, Yoon SK, et al. Phase IIb multicentred randomised trial of besifovir (LB80380) versus entecavir in Asian patients with chronic hepatitis B. Gut 2014;63:996-1004.


399. Gane E, Seto WK, Janssen H, Caruntu FA, Kim HJ, Abdurakhmanov D, et al. Safety and efficacy at 1 year after switching from Tenofovir Disoproxil Fumurate to Tenofovir Alafenamide in chronic HBV patients with risk factors for TDF use [Abstract]. J Hepatol 2018;68(S1):S87.

400. Yuki N, Nagaoka T, Yamashiro M, Mochizuki K, Kaneko A, Yamamoto K, et al. Long-term histologic and virologic outcomes of acute self-limited hepatitis B. Hepatology 2003;37:1172-1179.


401. Shiffman ML. Management of acute hepatitis B. Clin Liver Dis 2010;14:75-91 viii-ix.


402. Lampertico P, Maini M, Papatheodoridis G. Optimal management of hepatitis B virus infection - EASL Special Conference. J Hepatol 2015;63:1238-1253.


403. European Association for the Study of the Liver. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol 2017;66:1047-1081.


405. Mantzoukis K, Rodr├Łguez-Per├Īlvarez M, Buzzetti E, Thorburn D, Davidson BR, Tsochatzis E, et al. Pharmacological interventions for acute hepatitis B infection: an attempted network meta-analysis. Cochrane Database Syst Rev 2017;3:CD011645.

406. Kumar M, Satapathy S, Monga R, Das K, Hissar S, Pande C, et al. A randomized controlled trial of lamivudine to treat acute hepatitis B. Hepatology 2007;45:97-101.


409. Tillmann HL, Hadem J, Leifeld L, Zachou K, Canbay A, Eisenbach C, et al. Safety and efficacy of lamivudine in patients with severe acute or fulminant hepatitis B, a multicenter experience. J Viral Hepat 2006;13:256-263.


410. Miyake Y, Iwasaki Y, Takaki A, Fujioka S, Takaguchi K, Ikeda H, et al. Lamivudine treatment improves the prognosis of fulminant hepatitis B. Intern Med 2008;47:1293-1299.


411. Streinu-Cercel A, Sandulescu O, Stefan M, Streinu-Cercel A. Treatment with lamivudine and entecavir in severe acute hepatitis B. Indian J Med Microbiol 2016;34:166-172.


413. Gupta S, Govindarajan S, Fong TL, Redeker AG. Spontaneous reactivation in chronic hepatitis B: patterns and natural history. J Clin Gastroenterol 1990;12:562-568.


414. Lok AS, McMahon BJ; Practice Guidelines Committee, American Association for the Study of Liver Diseases (AASLD). Chronic hepatitis B: update of recommendations. Hepatology 2004;39:857-861.


415. Tanaka Y, Esumi M, Shikata T. Persistence of hepatitis B virus DNA after serological clearance of hepatitis B virus. Liver 1990;10:6-10.


416. Koo YX, Tan DS, Tan IB, Tao M, Chow WC, Lim ST. Hepatitis B virus reactivation and role of antiviral prophylaxis in lymphoma patients with past hepatitis B virus infection who are receiving chemoimmunotherapy. Cancer 2010;116:115-121.


418. Yeo W, Johnson PJ. Diagnosis, prevention and management of hepatitis B virus reactivation during anticancer therapy. Hepatology 2006;43:209-220.


421. Lok AS, Liang RH, Chiu EK, Wong KL, Chan TK, Todd D. Reactivation of hepatitis B virus replication in patients receiving cytotoxic therapy. Report of a prospective study. Gastroenterology 1991;100:182-188.


422. Yeo W, Chan PK, Zhong S, Ho WM, Steinberg JL, Tam JS, et al. Frequency of hepatitis B virus reactivation in cancer patients undergoing cytotoxic chemotherapy: a prospective study of 626 patients with identification of risk factors. J Med Virol 2000;62:299-307.


424. Kwak LW, Halpern J, Olshen RA, Horning SJ. Prognostic significance of actual dose intensity in diffuse large-cell lymphoma: results of a tree-structured survival analysis. J Clin Oncol 1990;8:963-977.


425. Bonadonna G, Valagussa P, Moliterni A, Zambetti M, Brambilla C. Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: the results of 20 years of follow-up. N Engl J Med 1995;332:901-906.


426. Yeo W, Chan PK, Hui P, Ho WM, Lam KC, Kwan WH, et al. Hepatitis B virus reactivation in breast cancer patients receiving cytotoxic chemotherapy: a prospective study. J Med Virol 2003;70:553-561.


428. Perrillo RP, Gish R, Falck-Ytter YT. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology 2015;148:221-244 e3.


429. Reddy KR, Beavers KL, Hammond SP, Lim JK, Falck-Ytter YT; American Gastroenterological Association Institute. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology 2015;148:215-219 quiz e16- e17.


430. Takai S, Tsurumi H, Ando K, Kasahara S, Sawada M, Yamada T, et al. Prevalence of hepatitis B and C virus infection in haematological malignancies and liver injury following chemotherapy. Eur J Haematol 2005;74:158-165.


431. Hsu C, Hsiung CA, Su IJ, Hwang WS, Wang MC, Lin SF, et al. A revisit of prophylactic lamivudine for chemotherapy-associated hepatitis B reactivation in non-HodgkinŌĆÖs lymphoma: a randomized trial. Hepatology 2008;47:844-853.


433. Cheng AL, Hsiung CA, Su IJ, Chen PJ, Chang MC, Tsao CJ, et al. Steroid-free chemotherapy decreases risk of hepatitis B virus (HBV) reactivation in HBV-carriers with lymphoma. Hepatology 2003;37:1320-1328.


434. Yeo W, Chan TC, Leung NW, Lam WY, Mo FK, Chu MT, et al. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol 2009;27:605-611.


435. Dong HJ, Ni LN, Sheng GF, Song HL, Xu JZ, Ling Y. Risk of hepatitis B virus (HBV) reactivation in non-Hodgkin lymphoma patients receiving rituximab-chemotherapy: a meta-analysis. J Clin Virol 2013;57:209-214.


437. Zurawska U, Hicks LK, Woo G, Bell CM, Krahn M, Chan KK, et al. Hepatitis B virus screening before chemotherapy for lymphoma: a cost-effectiveness analysis. J Clin Oncol 2012;30:3167-3173.


439. Lau GK, He ML, Fong DY, Bartholomeusz A, Au WY, Lie AK, et al. Preemptive use of lamivudine reduces hepatitis B exacerbation after allogeneic hematopoietic cell transplantation. Hepatology 2002;36:702-709.


440. Sarmati L, Andreoni M, Antonelli G, Arcese W, Bruno R, Coppola N, et al. Recommendations for screening, monitoring, prevention, prophylaxis and therapy of hepatitis B virus reactivation in patients with haematologic malignancies and patients who underwent haematologic stem cell transplantation-a position paper. Clin Microbiol Infect 2017;23:935-940.


442. Chen FW, Coyle L, Jones BE, Pattullo V. Entecavir versus lamivudine for hepatitis B prophylaxis in patients with haematological disease. Liver Int 2013;33:1203-1210.


444. Dai MS, Wu PF, Shyu RY, Lu JJ, Chao TY. Hepatitis B virus reactivation in breast cancer patients undergoing cytotoxic chemotherapy and the role of preemptive lamivudine administration. Liver Int 2004;24:540-546.


446. Xu Z, Dai W, Wu YT, Arshad B, Li X, Wu H, et al. Prophylactic effect of lamivudine on chemotherapy-induced hepatitis B virus reactivation in patients with solid tumour: a meta-analysis. Eur J Cancer Care (Engl) 2018;27:e12799.


447. El-Sayed MH, Mohamed MM, Karim A, Maina AM, Oliveri F, Brunetto MR, et al. Severe liver disease is caused by HBV rather than HCV in children with hematological malignancies. Hematol J 2003;4:321-327.


448. Yeo W, Chan PK, Ho WM, Zee B, Lam KC, Lei KI, et al. Lamivudine for the prevention of hepatitis B virus reactivation in hepatitis B santigen seropositive cancer patients undergoing cytotoxic chemotherapy. J Clin Oncol 2004;22:927-934.


449. Chung SJ, Kim JK, Park MC, Park YB, Lee SK. Reactivation of hepatitis B viral infection in inactive HBsAg carriers following anti-tumor necrosis factor-alpha therapy. J Rheumatol 2009;36:2416-2420.


452. Lee YH, Bae SC, Song GG. Hepatitis B virus reactivation in HBsAgpositive patients with rheumatic diseases undergoing anti-tumor necrosis factor therapy or DMARDs. Int J Rheum Dis 2013;16:527-531.


453. P├®rez-Alvarez R, D├Łaz-Lagares C, Garc├Ła-Hern├Īndez F, Lopez-Roses L, Brito-Zer├│n P, P├®rez-de-Lis M, et al. Hepatitis B virus (HBV) reactivation in patients receiving tumor necrosis factor (TNF)-targeted therapy: analysis of 257 cases. Medicine (Baltimore) 2011;90:359-371.


454. Lau GK, Yiu HH, Fong DY, Cheng HC, Au WY, Lai LS, et al. Early is superior to deferred preemptive lamivudine therapy for hepatitis B patients undergoing chemotherapy. Gastroenterology 2003;125:1742-1749.


456. Vigan├▓ M, Serra G, Casella G, Grossi G, Lampertico P. Reactivation of hepatitis B virus during targeted therapies for cancer and immune-mediated disorders. Expert Opin Biol Ther 2016;16:917-926.


458. Cerva C, Colagrossi L, Maffongelli G, Salpini R, Di Carlo D, Malagnino V, et al. Persistent risk of HBV reactivation despite extensive lamivudine prophylaxis in haematopoietic stem cell transplant recipients who are anti-HBc-positive or HBV-negative recipients with an anti-HBc-positive donor. Clin Microbiol Infect 2016;22:946 e1-946.e8.

459. Liu WP, Wang XP, Zheng W, Ping LY, Zhang C, Wang GQ, et al. Hepatitis B virus reactivation after withdrawal of prophylactic antiviral therapy in patients with diffuse large B cell lymphoma. Leuk Lymphoma 2016;57:1355-1362.


460. Nakaya A, Fujita S, Satake A, Nakanishi T, Azuma Y, Tsubokura Y, et al. Delayed HBV reactivation in rituximab-containing chemotherapy: how long should we continue anti-virus prophylaxis or monitoring HBV-DNA? Leuk Res 2016;50:46-49.


462. Rossi G, Pelizzari A, Motta M, Puoti M. Primary prophylaxis with lamivudine of hepatitis B virus reactivation in chronic HbsAg carriers with lymphoid malignancies treated with chemotherapy. Br J Haematol 2001;115:58-62.


463. Li HR, Huang JJ, Guo HQ, Zhang X, Xie Y, Zhu HL, et al. Comparison of entecavir and lamivudine in preventing hepatitis B reactivation in lymphoma patients during chemotherapy. J Viral Hepat 2011;18:877-883.


465. Demetris AJ, Jaffe R, Sheahan DG, Burnham J, Spero J, Iwatsuki S, et al. Recurrent hepatitis B in liver allograft recipients. Differentiation between viral hepatitis B and rejection. Am J Pathol 1986;125:161-172.


466. Demetris AJ, Todo S, Van Thiel DH, Fung JJ, Iwaki Y, Sysyn G, et al. Evolution of hepatitis B virus liver disease after hepatic replacement. Practical and theoretical considerations. Am J Pathol 1990;137:667-676.


467. Freeman RB, Sanchez H, Lewis WD, Sherburne B, Dzik WH, Khettry U, et al. Serologic and DNA follow-up data from HBsAg-positive patients treated with orthotopic liver transplantation. Transplantation 1991;51:793-797.


468. Lake JR, Wright TL. Liver transplantation for patients with hepatitis B: what have we learned from our results? Hepatology 1991;13:796-799.


469. OŌĆÖGrady JG, Smith HM, Davies SE, Daniels HM, Donaldson PT, Tan KC, et al. Hepatitis B virus reinfection after orthotopic liver transplantation. Serological and clinical implications. J Hepatol 1992;14:104-111.


470. Rizzetto M, Recchia S, Salizzoni M. Liver transplantation in carriers of the HBsAg. J Hepatol 1991;13:5-7.


472. Samuel D, Muller R, Alexander G, Fassati L, Ducot B, Benhamou JP, et al. Liver transplantation in European patients with the hepatitis B surface antigen. N Engl J Med 1993;329:1842-1847.


473. Han SH, Ofman J, Holt C, King K, Kunder G, Chen P, et al. An efficacy and cost-effectiveness analysis of combination hepatitis B immune globulin and lamivudine to prevent recurrent hepatitis B after orthotopic liver transplantation compared with hepatitis B immune globulin monotherapy. Liver Transpl 2000;6:741-748.


474. Markowitz JS, Martin P, Conrad AJ, Markmann JF, Seu P, Yersiz H, et al. Prophylaxis against hepatitis B recurrence following liver transplantation using combination lamivudine and hepatitis B immune globulin. Hepatology 1998;28:585-589.


475. Marzano A, Salizzoni M, Debernardi-Venon W, Smedile A, Franchello A, Ciancio A, et al. Prevention of hepatitis B virus recurrence after liver transplantation in cirrhotic patients treated with lamivudine and passive immunoprophylaxis. J Hepatol 2001;34:903-910.


476. Steinm├╝ller T, Seehofer D, Rayes N, M├╝ller AR, Settmacher U, Jonas S, et al. Increasing applicability of liver transplantation for patients with hepatitis B-related liver disease. Hepatology 2002;35:1528-1535.


477. Katz LH, Paul M, Guy DG, Tur-Kaspa R. Prevention of recurrent hepatitis B virus infection after liver transplantation: hepatitis B immunoglobulin, antiviral drugs, or both? Systematic review and meta-analysis. Transpl Infect Dis 2010;12:292-308.


479. Mutimer D, Dusheiko G, Barrett C, Grellier L, Ahmed M, Anschuetz G, et al. Lamivudine without HBIg for prevention of graft reinfection by hepatitis B: long-term follow-up. Transplantation 2000;70:809-815.


480. Perrillo RP, Wright T, Rakela J, Levy G, Schiff E, Gish R, et al. A multicenter United States-Canadian trial to assess lamivudine monotherapy before and after liver transplantation for chronic hepatitis B. Hepatology 2001;33:424-432.


483. Fung J, Cheung C, Chan SC, Yuen MF, Chok KS, Sharr W, et al. Entecavir monotherapy is effective in suppressing hepatitis B virus after liver transplantation. Gastroenterology 2011;141:1212-1219.


484. Fung J, Wong T, Chok K, Chan A, Cheung TT, Dai JW, et al. Longterm outcomes of entecavir monotherapy for chronic hepatitis B after liver transplantation: results up to 8 years. Hepatology 2017;66:1036-1044.


485. Cholongitas E, Papatheodoridis GV. High genetic barrier nucleos(t) ide analogue(s) for prophylaxis from hepatitis B virus recurrence after liver transplantation: a systematic review. Am J Transplant 2013;13:353-362.


486. Gane EJ, Angus PW, Strasser S, Crawford DH, Ring J, Jeffrey GP, et al. Lamivudine plus low-dose hepatitis B immunoglobulin to prevent recurrent hepatitis B following liver transplantation. Gastroenterology 2007;132:931-937.


487. Cholongitas E, Goulis I, Antoniadis N, Fouzas I, Imvrios G, Papanikolaou V, et al. New nucleos(t)ide analogue monoprophylaxis after cessation of hepatitis B immunoglobulin is effective against hepatitis B recurrence. Transpl Int 2014;27:1022-1028.


488. Cholongitas E, Vasiliadis T, Antoniadis N, Goulis I, Papanikolaou V, Akriviadis E. Hepatitis B prophylaxis post liver transplantation with newer nucleos(t)ide analogues after hepatitis B immunoglobulin discontinuation. Transpl Infect Dis 2012;14:479-487.


489. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Liver transplantation. J Hepatol 2016;64:433-485.


491. Fox AN, Terrault NA. The option of HBIG-free prophylaxis against recurrent HBV. J Hepatol 2012;56:1189-1197.


493. Fern├Īndez I, Loinaz C, Hern├Īndez O, Abradelo M, Manrique A, Calvo J, et al. Tenofovir/entecavir monotherapy after hepatitis B immunoglobulin withdrawal is safe and effective in the prevention of hepatitis B in liver transplant recipients. Transpl Infect Dis 2015;17:695-701.


494. Cholongitas E, Papatheodoridis GV, Burroughs AK. Liver grafts from anti-hepatitis B core positive donors: a systematic review. J Hepatol 2010;52:272-279.


495. Wachs ME, Amend WJ, Ascher NL, Bretan PN, Emond J, Lake JR, et al. The risk of transmission of hepatitis B from HBsAg(-), HBcAb(+), HBIgM(-) organ donors. Transplantation 1995;59:230-234.


496. Prieto M, G├│mez MD, Berenguer M, C├│rdoba J, Ray├│n JM, Pastor M, et al. De novo hepatitis B after liver transplantation from hepatitis B core antibody-positive donors in an area with high prevalence of anti-HBc positivity in the donor population. Liver Transpl 2001;7:51-58.


498. Avelino-Silva VI, DŌĆÖAlbuquerque LA, Bonazzi PR, Song AT, Miraglia JL, De Brito Neves A, et al. Liver transplant from Anti-HBc-positive, HBsAg-negative donor into HBsAg-negative recipient: is it safe? A systematic review of the literature. Clin Transplant 2010;24:735-746.


499. Saab S, Waterman B, Chi AC, Tong MJ. Comparison of different immunoprophylaxis regimens after liver transplantation with hepatitis B core antibody-positive donors: a systematic review. Liver Transpl 2010;16:300-307.


500. Lee KW, Lee DS, Lee HH, Kim SJ, Joh JW, Seo JM, et al. Prevention of de novo hepatitis B infection from HbcAb-positive donors in living donor liver transplantation. Transplant Proc 2004;36:2311-2312.


502. Wright AJ, Fishman JA, Chung RT. Lamivudine compared with newer antivirals for prophylaxis of hepatitis B core antibody positive livers: a cost-effectiveness analysis. Am J Transplant 2014;14:629-634.


503. Fontana RJ, Hann HW, Wright T, Everson G, Baker A, Schiff ER, et al. A multicenter study of lamivudine treatment in 33 patients with hepatitis B after liver transplantation. Liver Transpl 2001;7:504-510.


504. Ben-Ari Z, Mor E, Shapira Z, Tur-Kaspa R. Long-term experience with lamivudine therapy for hepatitis B virus infection after liver transplantation. Liver Transpl 2001;7:113-117.


505. Perrillo R, Rakela J, Dienstag J, Levy G, Martin P, Wright T, et al. Multicenter study of lamivudine therapy for hepatitis B after liver transplantation. Lamivudine Transplant Group. Hepatology 1999;29:1581-1586.


506. Ben-Ari Z, Pappo O, Zemel R, Mor E, Tur-Kaspa R. Association of lamivudine resistance in recurrent hepatitis B after liver transplantation with advanced hepatic fibrosis. Transplantation 1999;68:232-236.


507. Neff GW, OŌĆÖBrien CB, Nery J, Shire N, Montalbano M, Ruiz P, et al. Outcomes in liver transplant recipients with hepatitis B virus: resistance and recurrence patterns from a large transplant center over the last decade. Liver Transpl 2004;10:1372-1378.


508. Fornairon S, Pol S, Legendre C, Carnot F, Mamzer-Bruneel MF, Brechot C, et al. The long-term virologic and pathologic impact of renal transplantation on chronic hepatitis B virus infection. Transplantation 1996;62:297-299.


509. Ahn HJ, Kim MS, Kim YS, Kim SI, Huh KH, Ju MK, et al. Clinical outcome of renal transplantation in patients with positive pretransplant hepatitis B surface antigen. J Med Virol 2007;79:1655-1663.


510. Cosconea S, Fontaine H, M├®ritet JF, Corouge M, Sogni P, ValletPichard A, et al. Benefits associated with antiviral treatment in kidney allograft recipients with chronic hepatitis B virus infection. J Hepatol 2012;57:55-60.


511. Yap DY, Tang CS, Yung S, Choy BY, Yuen MF, Chan TM. Longterm outcome of renal transplant recipients with chronic hepatitis B infection-impact of antiviral treatments. Transplantation 2010;90:325-330.


512. Yap DY, Yung S, Tang CS, Seto WK, Ma MK, Mok MM, et al. Entecavir treatment in kidney transplant recipients infected with hepatitis B. Clin Transplant 2014;28:1010-1015.


514. Shin HS, Cho HJ, Jeon ES, Hwang HY, Kim JJ, Kim KB, et al. The impact of hepatitis B on heart transplantation: 19 years of national experience in Korea. Ann Transplant 2014;19:182-187.


515. Durante-Mangoni E, Vitrone M, Parrella A, Andini R, Iossa D, Ragone E, et al. Efficacy and safety of tenofovir, entecavir, and telbivudine for chronic hepatitis B in heart transplant recipients. Transpl Infect Dis 2016;18:319-325.


516. Jeon JW, Kim SM, Cho H, Baek CH, Kim H, Shin S, et al. Presence of hepatitis B surface antibody in addition to hepatitis B core antibody confers protection against hepatitis B virus infection in hepatitis B surface antigen-negative patients undergoing kidney transplantation. Transplantation 2018;102:1717-1723.


518. Masutani K, Omoto K, Okumi M, Okabe Y, Shimizu T, Tsuruya K, et al. Incidence of hepatitis B viral reactivation after kidney transplantation with low-dose rituximab administration. Transplantation 2018;102:140-145.


519. Mahboobi N, Tabatabaei SV, Blum HE, Alavian SM. Renal grafts from anti-hepatitis B core-positive donors: a quantitative review of the literature. Transpl Infect Dis 2012;14:445-451.


520. Chamorro C, Aparicio M. Influence of anti-HBc positive organ donor in lung donor selection. Arch Bronconeumol 2012;48:320-324.


521. Huprikar S, Danziger-Isakov L, Ahn J, Naugler S, Blumberg E, Avery RK, et al. Solid organ transplantation from hepatitis B virus-positive donors: consensus guidelines for recipient management. Am J Transplant 2015;15:1162-1172.


522. Lau GK, Leung YH, Fong DY, Au WY, Kwong YL, Lie A, et al. High hepatitis B virus (HBV) DNA viral load as the most important risk factor for HBV reactivation in patients positive for HBV surface antigen undergoing autologous hematopoietic cell transplantation. Blood 2002;99:2324-2330.


526. Seto WK, Chan TS, Hwang YY, Wong DK, Fung J, Liu KS, et al. Hepatitis B reactivation in occult viral carriers undergoing hematopoietic stem cell transplantation: a prospective study. Hepatology 2017;65:1451-1461.


529. Lee LP, Dai CY, Chuang WL, Chang WY, Hou NJ, Hsieh MY, et al. Comparison of liver histopathology between chronic hepatitis C patients and chronic hepatitis B and C-coinfected patients. J Gastroenterol Hepatol 2007;22:515-517.


530. Pol S, Haour G, Fontaine H, Dorival C, Petrov-Sanchez V, Bourliere M, et al. The negative impact of HBV/HCV coinfection on cirrhosis and its consequences. Aliment Pharmacol Ther 2017;46:1054-1060.


531. Chen G, Wang C, Chen J, Ji D, Wang Y, Wu V, et al. Hepatitis B reactivation in hepatitis B and C coinfected patients treated with antiviral agents: a systematic review and meta-analysis. Hepatology 2017;66:13-26.


532. Liu CJ, Chuang WL, Sheen IS, Wang HY, Chen CY, Tseng KC, et al. Efficacy of ledipasvir and sofosbuvir treatment of HCV infection in patients coinfected with HBV. Gastroenterology 2018;154:989-997.


533. Bersoff-Matcha SJ, Cao K, Jason M, Ajao A, Jones SC, Meyer T, et al. Hepatitis B virus reactivation associated with direct-acting antiviral therapy for chronic hepatitis C virus: a review of cases reported to the U.S. Food and Drug Administration adverse event reporting system. Ann Intern Med 2017;166:792-798.


535. Thio CL, Seaberg EC, Skolasky R Jr, Phair J, Visscher B, Mu├▒oz A, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet 2002;360:1921-1926.


537. World Health Organization (WHO). Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2nd ed. Geneva: WHO; 2016. p. 208.
538. Wranke A, Pinheiro Borzacov LM, Parana R, Lobato C, Hamid S, Ceausu E, et al. Clinical and virological heterogeneity of hepatitis delta in different regions world-wide: the Hepatitis Delta International Network (HDIN). Liver Int 2018;38:842-850.


539. Jeong SH, Kim JM, Ahn HJ, Park MJ, Paik KH, Choi W, et al. The prevalence and clinical characteristics of hepatitis-delta infection in Korea. Korean J Hepatol 2005;11:43-50.

540. Kim HS, Kim SJ, Park HW, Shin WG, Kim KH, Lee JH, et al. Prevalence and clinical significance of hepatitis D virus coinfection in patients with chronic hepatitis B in Korea. J Med Virol 2011;83:1172-1177.


541. Bahcecioglu IH, Aygun C, Gozel N, Poyrazoglu OK, Bulut Y, Yalniz M. Prevalence of hepatitis delta virus (HDV) infection in chronic hepatitis B patients in eastern Turkey: still a serious problem to consider. J Viral Hepat 2011;18:518-524.


543. Triantos C, Kalafateli M, Nikolopoulou V, Burroughs A. Metaanalysis: antiviral treatment for hepatitis D. Aliment Pharmacol Ther 2012;35:663-673.


544. Wedemeyer H, Yurdayd├¼n C, Dalekos GN, Erhardt A, ├ćakalo─¤lu Y, De─¤ertekin H, et al. Peginterferon plus adefovir versus either drug alone for hepatitis delta. N Engl J Med 2011;364:322-331.


545. Abbas Z, Memon MS, Mithani H, Jafri W, Hamid S. Treatment of chronic hepatitis D patients with pegylated interferon: a real-world experience. Antivir Ther 2014;19:463-468.


546. Keskin O, Wedemeyer H, T├╝z├╝n A, Zachou K, Deda X, Dalekos GN, et al. Association between level of hepatitis D virus RNA at week 24 of pegylated interferon therapy and outcome. Clin Gastroenterol Hepatol 2015;13:2342-2349 e1-e2.


547. Heidrich B, Yurdayd─▒n C, Kaba├¦am G, Ratsch BA, Zachou K, Bremer B, et al. Late HDV RNA relapse after peginterferon alpha-based therapy of chronic hepatitis delta. Hepatology 2014;60:87-97.


548. Karaca C, Soyer OM, Baran B, Ormeci AC, Gokturk S, Aydin E, et al. Efficacy of pegylated interferon-╬▒ treatment for 24 months in chronic delta hepatitis and predictors of response. Antivir Ther 2013;18:561-566.


549. S├Čderstr├Čm A, Norkrans G, Lindh M. Hepatitis B virus DNA during pregnancy and post partum: aspects on vertical transmission. Scand J Infect Dis 2003;35:814-819.


550. ter Borg MJ, Leemans WF, de Man RA, Janssen HL. Exacerbation of chronic hepatitis B infection after delivery. J Viral Hepat 2008;15:37-41.


551. Samadi Kochaksaraei G, Castillo E, Osman M, Simmonds K, Scott AN, Oshiomogho JI, et al. Clinical course of 161 untreated and tenofovir-treated chronic hepatitis B pregnant patients in a low hepatitis B virus endemic region. J Viral Hepat 2016;23:15-22.


553. Fontana RJ. Side effects of long-term oral antiviral therapy for hepatitis B. Hepatology 2009;49(5 Suppl):S185-S195.


556. Nguyen G, Garcia RT, Nguyen N, Trinh H, Keeffe EB, Nguyen MH. Clinical course of hepatitis B virus infection during pregnancy. Aliment Pharmacol Ther 2009;29:755-764.


557. Chang CY, Aziz N, Poongkunran M, Javaid A, Trinh HN, Lau DT, et al. Serum aminotransferase flares in pregnant and postpartum women with current or prior treatment for chronic hepatitis B. J Clin Gastroenterol 2018;52:255-261.


558. Zhang L, Gui X, Fan J, Wang B, Ji H, Yisilafu R, et al. Breast feeding and immunoprophylaxis efficacy of mother-to-child transmission of hepatitis B virus. J Matern Fetal Neonatal Med 2014;27:182-186.


562. World Health Organization (WHO). WHO Technical brief: Preventing HIV during pregnancy and breastfeeding in the context of preexposure prophylaxis (PrEP). Geneva: WHO; 2017. p. 10.
563. Wen WH, Chang MH, Zhao LL, Ni YH, Hsu HY, Wu JF, et al. Motherto-infant transmission of hepatitis B virus infection: significance of maternal viral load and strategies for intervention. J Hepatol 2013;59:24-30.


564. Zou H, Chen Y, Duan Z, Zhang H, Pan C. Virologic factors associated with failure to passive-active immunoprophylaxis in infants born to HBsAg-positive mothers. J Viral Hepat 2012;19:e18-e25.


566. Pan CQ, Han GR, Jiang HX, Zhao W, Cao MK, Wang CM, et al. Telbivudine prevents vertical transmission from HBeAg-positive women with chronic hepatitis B. Clin Gastroenterol Hepatol 2012;10:520-526.


567. Chen HL, Lee CN, Chang CH, Ni YH, Shyu MK, Chen SM, et al. Efficacy of maternal tenofovir disoproxil fumarate in interrupting mother-to-infant transmission of hepatitis B virus. Hepatology 2015;62:375-386.


568. Pan CQ, Duan Z, Dai E, Zhang S, Han G, Wang Y, et al. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med 2016;374:2324-2334.


569. Hyun MH, Lee YS, Kim JH, Je JH, Yoo YJ, Yeon JE, et al. Systematic review with meta-analysis: the efficacy and safety of tenofovir to prevent mother-to-child transmission of hepatitis B virus. Aliment Pharmacol Ther 2017;45:1493-1505.


571. Kim JH, Lee YS, Lee HS, Chang SW, Yeon JE, Byun KS. The role of tenofovir for prevention of vertical transmission of hepatitis B virus: systematic review with meta-analysis [Abstract]. Gut Liver 2018;12(Suppl 1):S109.
574. Zhu S, Zhang H, Dong Y, Wang L, Xu Z, Liu W, et al. Antiviral therapy in hepatitis B virus-infected children with immune-tolerant characteristics: a pilot open-label randomized study. J Hepatol 2018;68:1123-1128.


575. Jonas MM, Block JM, Haber BA, Karpen SJ, London WT, Murray KF, et al. Treatment of children with chronic hepatitis B virus infection in the United States: patient selection and therapeutic options. Hepatology 2010;52:2192-2205.


576. Sokal EM, Conjeevaram HS, Roberts EA, Alvarez F, Bern EM, Goyens P, et al. Interferon alfa therapy for chronic hepatitis B in children: a multinational randomized controlled trial. Gastroenterology 1998;114:988-995.


577. Kobak GE, MacKenzie T, Sokol RJ, Narkewicz MR. Interferon treatment for chronic hepatitis B: enhanced response in children 5 years old or younger. J Pediatr 2004;145:340-345.


578. Wirth S, Zhang H, Hardikar W, Schwarz KB, Sokal E, Yang W, et al. Efficacy and safety of peginterferon alfa-2a (40KD) in children with chronic hepatitis B: the PEG-B-ACTIVE study. Hepatology 2018;68:1681-1694.


579. Jonas MM, Chang MH, Sokal E, Schwarz KB, Kelly D, Kim KM, et al. Randomized, controlled trial of entecavir versus placebo in children with hepatitis B envelope antigen-positive chronic hepatitis B. Hepatology 2016;63:377-387.


581. Murray KF, Szenborn L, Wysocki J, Rossi S, Corsa AC, Dinh P, et al. Randomized, placebo-controlled trial of tenofovir disoproxil fumarate in adolescents with chronic hepatitis B. Hepatology 2012;56:2018-2026.


583. Jonas MM, Mizerski J, Badia IB, Areias JA, Schwarz KB, Little NR, et al. Clinical trial of lamivudine in children with chronic hepatitis B. N Engl J Med 2002;346:1706-1713.


584. Sokal EM, Kelly DA, Mizerski J, Badia IB, Areias JA, Schwarz KB, et al. Long-term lamivudine therapy for children with HBeAg-positive chronic hepatitis B. Hepatology 2006;43:225-232.


585. Choe BH, Lee JH, Jang YC, Jang CH, Oh KW, Kwon S, et al. Longterm therapeutic efficacy of lamivudine compared with interferonalpha in children with chronic hepatitis B: the younger the better. J Pediatr Gastroenterol Nutr 2007;44:92-98.


587. Chu M, Cho SM, Choe BH, Cho MH, Kwon S, Lee WK. Virologic responses to add-on adefovir dipivoxil treatment versus entecavir monotherapy in children with lamivudine-resistant chronic hepatitis B. J Pediatr Gastroenterol Nutr 2012;55:648-652.


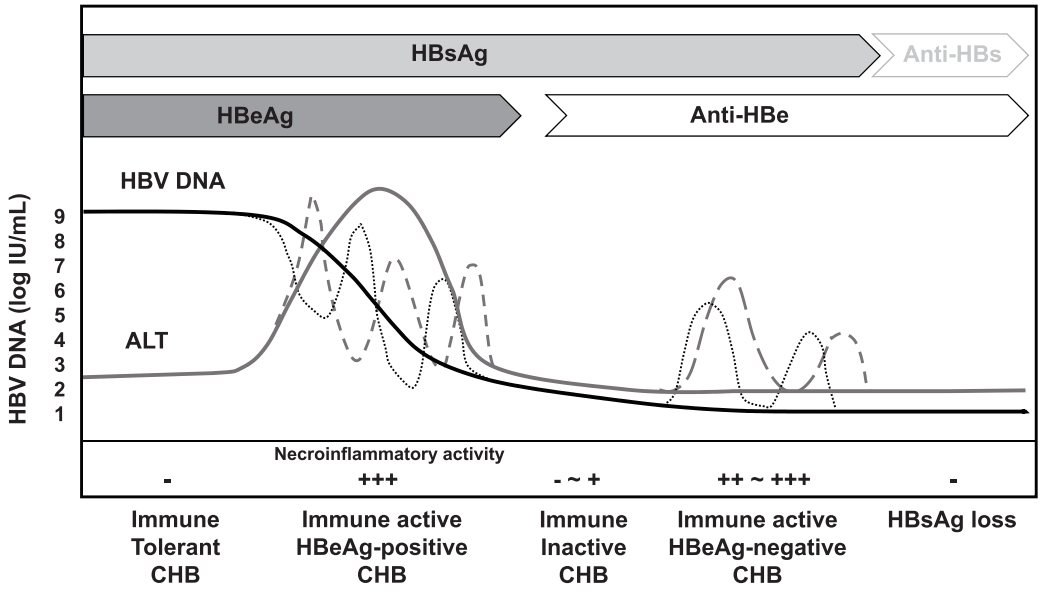
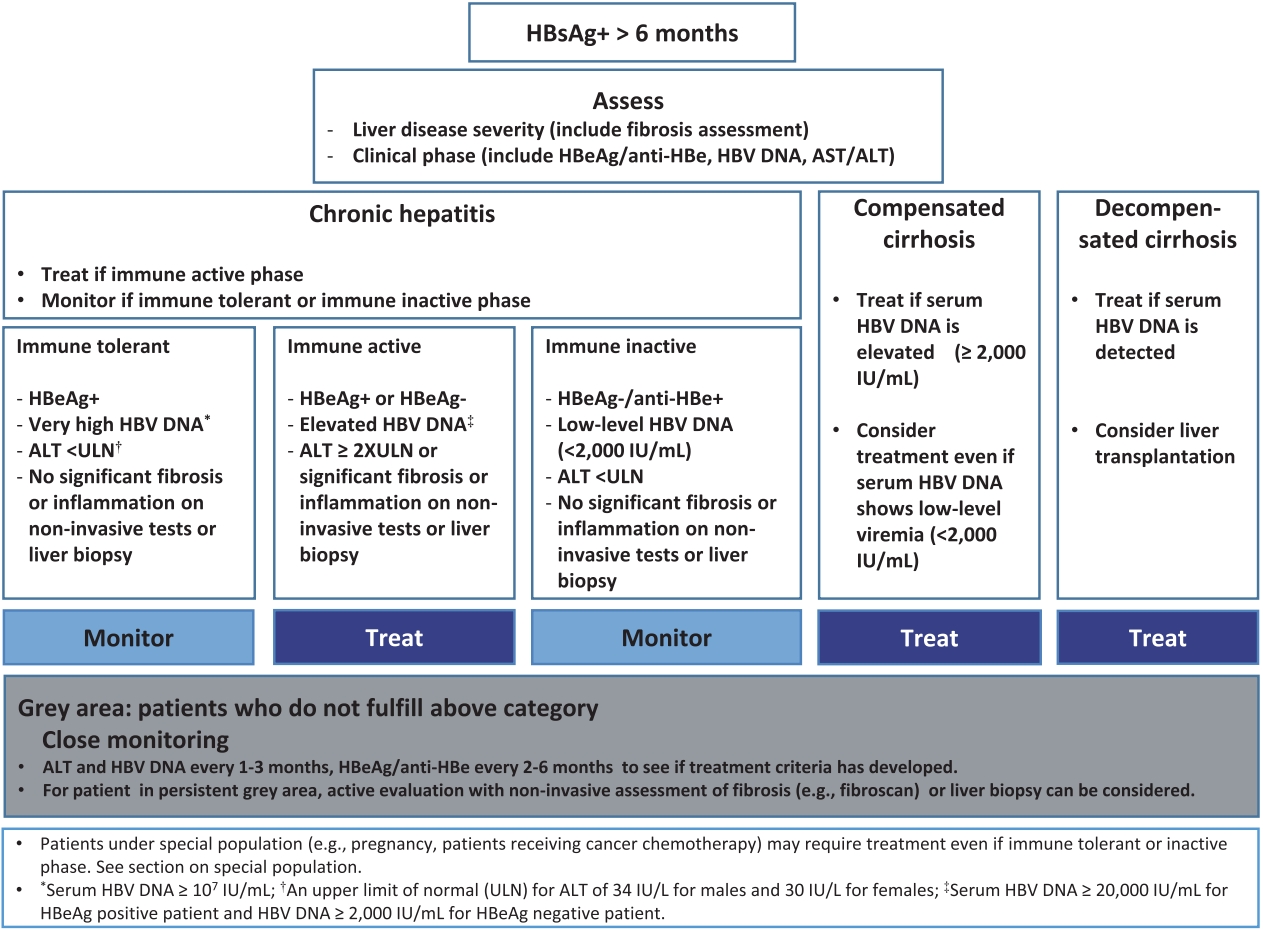
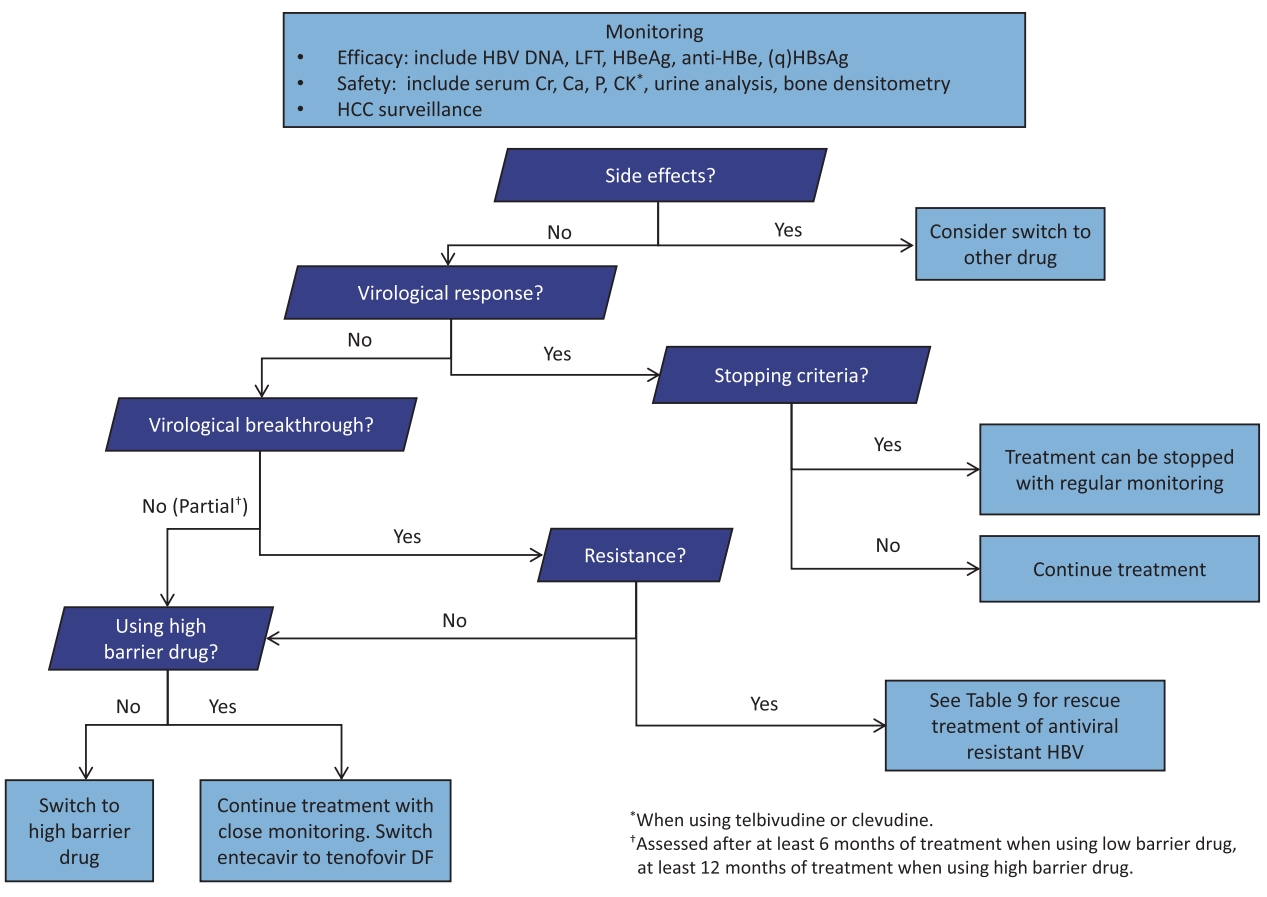
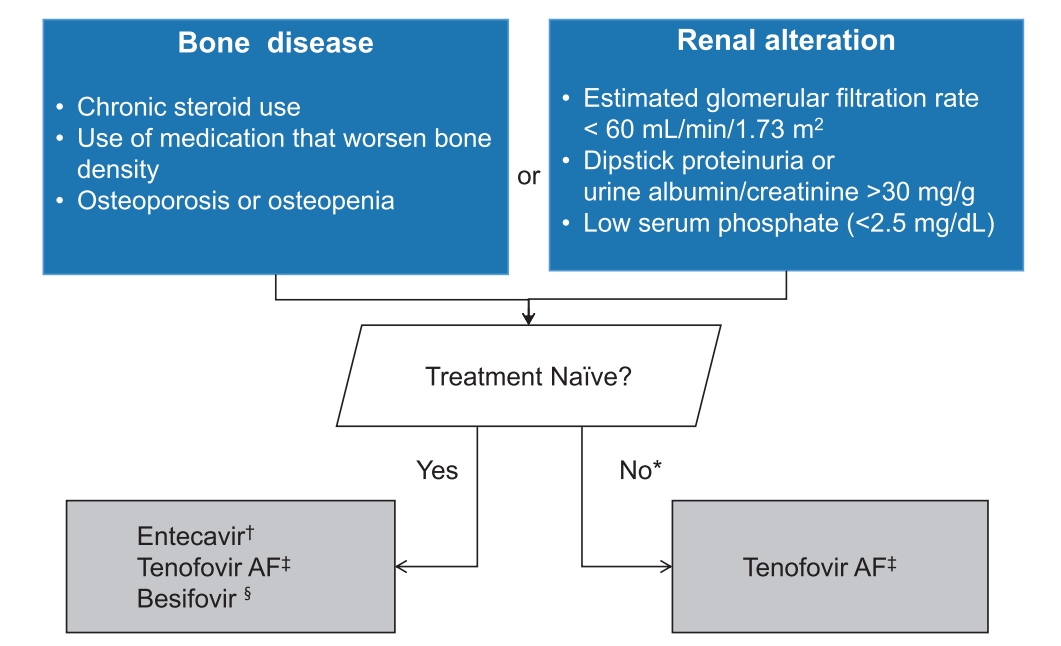




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Supplement
Supplement Print
Print



