MAFLD: How is it different from NAFLD?
Article information
Abstract
“Metabolic dysfunction-associated fatty liver disease (MAFLD)” is the term suggested in 2020 to refer to fatty liver disease related to systemic metabolic dysregulation. The name change from nonalcoholic fatty liver disease (NAFLD) to MAFLD comes with a simple set of criteria to enable easy diagnosis at the bedside for the general medical community, including primary care physicians. Since the introduction of the term, there have been key areas in which the superiority of MAFLD over the traditional NAFLD terminology has been demonstrated, including for the risk of liver and extrahepatic mortality, disease associations, and for identifying high-risk individuals. Additionally, MAFLD has been adopted by a number of leading pan-national and national societies due to its concise diagnostic criterion, removal of the requirement to exclude concomitant liver diseases, and reduction in the stigma associated with this condition. The current article explores the differences between MAFLD and NAFLD diagnosis, areas of benefit, some potential limitations, and how the MAFLD terminology has opened up new fields of research.
INTRODUCTION
Excess fat deposition within the liver has been recognized for centuries. In a landmark paper published by Ludwig et al. [1] (1980), the term “non-alcoholic steatohepatitis (NASH)” was first used to describe the liver histology associated with excess liver fat in the absence of significant alcohol consumption. The term “non-alcoholic” used by the researchers was derived from similarities in the histopathological findings of these patients compared to those with alcohol-related liver disease, due to the lack of knowledge about its pathophysiological basis at that time [1].
Ever since the introduction of the term nonalcoholic fatty liver disease (NAFLD) into the medical compendium, there has been discussions around changing the name to better reflect the disease process and extending the terminology beyond the superficial histopathological similarities to alcohol-related liver disease [2,3]. In early 2020, an international panel of experts led a consensus-driven process to develop a more appropriate term for the disease. Utilizing a 2-stage Delphi consensus, the term that was proposed was “metabolic dysfunction-associated fatty liver disease,” or “MAFLD” [4].
In addition to the name change, the consensus proposed a set of simple positive criteria to diagnose and evaluate individuals for the disease [4]. The diagnostic criterion highlighted the contribution that systemic metabolic dysregulation plays in driving the liver disease (Fig. 1). These contributory factors have since been identified as core research in the field of “NAFLD” and its extra-hepatic associations [5].
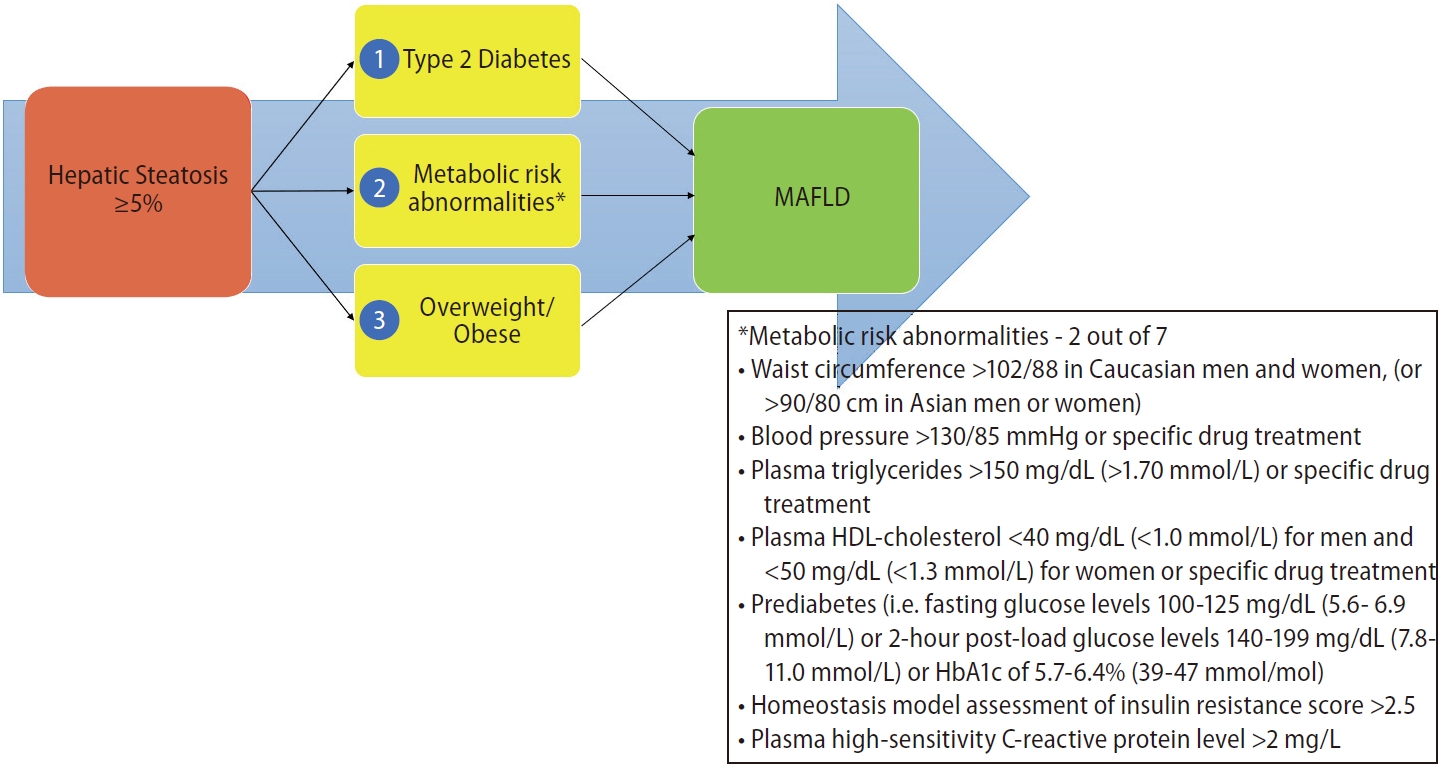
Diagnostic criterion for MAFLD. MAFLD, metabolic dysfunction-associated fatty liver disease; HDL, high-density lipoprotein.
Since the introduction of MAFLD in 2020 as an alternative term with its own set of diagnostic criteria, there have been more than 800 unique articles referencing the new diagnosis. There has also been controversy with some societies supporting its usage and introducing it as a formal change in terminology and diagnosis in their guidelines [6-10]. This article expands on the differences between MAFLD and NAFLD and the potential benefits and detriments of this change.
MAFLD VS. NAFLD – THE DIFFERENCES
NAFLD vs. MAFLD diagnosis – Criterion changes
The NAFLD diagnosis, as published in guidelines, requires hepatic steatosis of ≥5% without concurrent liver disease, including “significant” alcohol usage (Fig. 2) [11]. The criterion for MAFLD utilizes the same standard for hepatic steatosis, but identifies metabolic dysregulatory factors as a pre-requisite for the diagnosis to be entertained (Fig. 1) [4]. The metabolic risk drivers, according to the MAFLD criteria, are type 2 diabetes mellitus and overweight/obesity by ethnicspecific body mass index (BMI) classifications. Both of these risk factors are classically involved in liver fat deposition, and have been noted to be associated with an increase in disease progression and of hepatic and extra-hepatic complications. The third dysregulatory pathway is less commonly recognized but is part of the operational definition of metabolic syndrome. For the diagnosis of MAFLD in healthy weight people, an individual needs to have two of the seven risk factors to make a diagnosis. The risk factors include waist circumference, blood pressure, plasma triglycerides, plasma high-density lipoprotein-cholesterol, prediabetes, homeostasis model assessment of insulin resistance score, and plasma high sensitivity C-reactive protein. The combination of hepatic steatosis with one of these three metabolic risk stratifications results in the diagnosis of MAFLD [4].
The most significant difference between NAFLD and the diagnosis of MAFLD, however, is not the formal recognition of metabolic dysregulatory pathways in the development of the disease, but rather the removal of exclusion of concurrent liver disease to entertain the diagnosis [4,12]. Multiple studies have shown the synergistic effects of comorbid liver disease, including viral hepatitis, and concurrent alcohol usage; however, the exclusion of these in the diagnosis of NAFLD underpins a cognitive dissonance between these disease processes, attempting to exclude their contribution to individualized patient outcomes [13,14]. In short, MAFLD tells us what the disease is and not what it is not, and MAFLD is unrelated to the presence or absence of other causes of liver disease. This simple change has allowed clinicians to identify and treat all the liver diseases that might exist in a given patient in a holistic manner. The latter is important, given that in many countries and regions, overweight or obesity impacts over 60% of the adult population.
Positive diagnostic criterion versus negative diagnosis criterion
The switch to a set of positive diagnostic criteria results in the ability to detect all underlying liver diseases, particularly in patients without apparently clear metabolic features. A recent study by Alexander et al. [15] (2018) utilized multiple European primary care databases to determine the prevalence of NAFLD in general practice among 17.7 million patients. It found that the pooled prevalence of NAFLD was significantly lower than expected, with only 1.9% given this diagnosis in 2015 compared to prior observational studies estimating a prevalence of 20–30% in the European population. Although the prevalence has doubled since 2007 in these general practice databases, their recognition has not increased to meet the conservative estimates of this disorder.
In the age of improved investigations, there has been a significant move in most specialties to create positive diagnostic criterion for diseases. The benefits include decreased time to diagnosis and initiation of treatment, as well as consistent diagnosis facilitating both collaboration and research into the underlying disorders [4,16]. While this has occurred in a number of specialties, the prevalence of these disorders has been rare, and has not garnered the level of controversy that the proposed terminology of MAFLD has within the liver community. A recent example is the change from primary biliary cirrhosis to primary biliary cholangitis, which better reflects the pathophysiological manifestations of the disease process, as this condition has rarely resulted in cirrhosis.
For a disorder with a significant prevalence in the community, the NAFLD diagnostic criteria did not lead to increased understanding in the healthcare community and the wider general public. Fatty liver deposition is one of the most prevalent conditions affecting up to 40% of the general population; however, the recognition of the condition and its associated complications is poor [17]. One of the major controversies regarding the change in terminology to MAFLD is the suggestion that a name change will impair the current work to improve public awareness of NAFLD. However, the public awareness of NAFLD as a condition of concern is surprisingly low (around 4%), despite being included in the medical compendium for over 40 years [17]. There have been some suggestions that the change in name from NAFLD to MAFLD will increase public awareness of this condition [16,18,19].
Contributory factors to development of fatty liver disease
MAFLD diagnosis has been crucial in identifying higher-risk patients who would benefit from targeted management. Several studies have highlighted that a MAFLD diagnosis better correlates with higher liver fibrosis stage and non-invasive markers of fatty infiltration [20-23]. This recognition that metabolic dysregulatory pathways contribute to more significant liver disease highlights the important difference of the MAFLD diagnostic criteria over the NAFLD exclusionary criteria to assess individuals suffering from the disease.
NAFLD – A nebulous diagnosis
Despite the histopathological premise for the term NAFLD and advances in understanding the pathophysiological basis of the disease with many patient and healthcare suggestions for a name change, no new terminology has been developed and NAFLD has subsisted in the literature for decades [24]. Moreover, utilization of the diagnosis of NAFLD in healthcare outside of the gastroenterology specialty has been sparse. In a survey conducted by non-gastroenterology specialists in Australia, 56% of the respondents believed that NAFLD was related to alcohol intake [25]. This suggests that, despite non-alcoholic being the defining feature of the term “NAFLD” documented clearly within the name, the term is nebulous even among hospital specialists and not reflective of the practice need.
Another key characteristic of NAFLD is the exclusion of harmful alcohol intake in individuals with the disease. There are a number of reasons why harmful alcohol intake should not be used as an exclusionary tool in fatty liver disease. The first is that alcohol intake is a self-reported measure by a patient and has a variable designation of volume in different societal settings. Due to the stigma associated with alcohol consumption and its effects on the liver, under-reporting by patients has been identified [26]. A recent study performed by Staufer et al. [27] in 2022 also has also called into question the utilization of NAFLD after examining ethyl glucuronide in hair samples collected to assess alcohol consumption. In this prospective study, 114 patients were diagnosed with NAFLD after exclusion of other chronic liver diseases and alcohol consumption by patient recall. Harmful alcohol consumption was designated as >20 g of EtOH/day for women and >30 g of EtOH/day for men. The study found that 29% of the patients diagnosed with NAFLD had high to moderate risk of alcohol-related liver damage with repeated moderate to excessive alcohol consumption after being confronted with hair analysis, showing elevated levels of ethyl glucuronide. In a study directly assessing NAFLD diagnosis, almost 30% of the patients had elevated alcohol levels, which contradicted the basis for the diagnosis of NAFLD [27].
Confounding the picture even further is a recent paper by Meijnikman et al. [28] (2022) regarding the role of the gut microbiome in generating endogenous ethanol. In that study assessing obese NAFLD and NASH patients, portal vein and peripheral blood were taken to assess ethanol. It showed that microbiome-related ethanol production occurs in all populations, but was significantly higher in NASH and NAFLD when compared to patients without hepatic steatosis. This microbiome-induced ethanol production did not produce high peripheral concentrations of alcohol due to the livers’ ability to process large quantities of ethanol. The main point of this study was that, even though exogenous ethanol has been accounted for in the diagnostic terminology, there is a possibility that endogenous ethanol production by the microbiome could be contributory to its development [28]. Due to the histopathological similarities between alcohol-related liver disease and NAFLD, it is possible that the mechanism of injury is similar, but from different sources.
Secondly, there is heterogeneous reporting requirements across geographic regions governing the volume of alcohol considered to be harmful. Examples of this include the American Associated for the Study of Liver Disease and the Asian Pacific Association for the Study of the Liver guidelines, which define heavy or at-risk drinking as more than 14 drinks per week for men or more than seven drinks per week for women [11]. In the European Associated for the Study of the Liver guidelines, the diagnosis of NAFLD requires the exclusion of daily alcohol consumption of >30 g for men and >20 g for women [29]. Thirdly, even light or moderate alcohol consumption in the setting of NAFLD, which does not meet the exclusionary criteria set above, can cause significant worsening of fibrosis when compared to no consumption [30]. This has been shown in studies where even mild alcohol usage worsened fibrosis and may synergistically cause cirrhosis in patients diagnosed with NAFLD.
Due to the lack of histological characteristic features distinguishing alcohol-related fatty infiltration from non-alcohol-related fatty liver infiltration, the utilization of “non-alcoholic” via comprehensive alcohol assessment as a patient-reported measure with the associated stigmatization calls into question its ongoing use. This is particularly important, as international guidelines have recommended that “non-harmful” alcohol consumption has been shown to worsen fibrosis in patients with fatty liver disease. Additionally, evidence pointing towards increased endogenous ethanol production by the microbiome in fatty liver disease could be contributory to the underlying pathogenesis.
MAFLD VS. NAFLD – THE OVERALL BENEFITS
Identification of at-risk individuals
The utilization of previously collected databases to assess the applicability of MAFLD has been undertaken by several authors. The first of these studies performed by Lin et al. used the National Health and Nutrition Examination Surveys (NHANES) from 1988–1994, which examined 13,083 patients with complete ultrasonography and laboratory data [31]. Patients who met the MAFLD diagnostic criteria had statistically significant increases in metabolic comorbidities, liver enzymes, and non-invasive liver fibrosis scores compared to the NAFLD group.
A review performed by Kang et al. [32] in 2021 on behalf of the Korean NAFLD study group examined the publications that compared MAFLD to NAFLD, with a particular focus on the combined associations of risks in retrospective studies. It showed that MAFLD had statistically significant increases in alanine transferase (23.96±22.22 vs. 22.31±21.34, P≤0.001), NAFLD fibrosis score (–2.05±1.51 vs. –2.18±1.52, P≤0.001), and fibrosis-4 (FIB-4) scores (1.06±1.35 vs. 1.01±0.84, P≤0.001) compared to NAFLD. This indicates that MAFLD more specifically selects patients with worse liver function and non-invasive scores. These differences were even more striking in the comparison of MAFLD to non-metabolic risk (MR) NAFLD (or NAFLD patients without the necessary metabolic risk factors to meet the criteria for MAFLD). Utilizing MAFLD diagnostic criteria compared to non-MR NAFLD, the increases became more marked in alanine transferase (23.96±22.22 vs. 16.81±17.84, P≤0.001), NAFLD fibrosis score (–2.05±1.51 vs. –3.00±1.32, P≤0.001), and FIB-4 scores (1.06±1.35 vs. 0.87±1.05, P≤0.001). This highlights the utility of the MAFLD criteria over the traditional NAFLD diagnostic criteria in assessing patients for worsening liver disease. These analyses of large patient cohorts have also correctly identified most patients who have higher related risks for comorbidities and increased mortality. For example, the diagnosis of MAFLD has been shown to be superior in identifying patients who are most at risk for clinical disease progression compared to NAFLD [21,33].
Public awareness
There is limited historical evidence for the recognition of NAFLD and its contributory factors in the literature. Evidence that is available suggests that NAFLD recognition and diagnosis in primary care settings that manage the majority of patients are poorly understood and applied [15,25,31,34,35]. The simple criteria for MAFLD have been purported to increase the recognition and understanding outside of gastroenterology and hepatology specialists, and it will also enable primary care practitioners and others to initiate early management [16,18,19,36]. This has not been studied in the literature to date, but would be significant to public health as early interventions, similar to cardiovascular disease and diabetes mellitus, are more likely to be efficacious in preventing adverse outcomes.
From an individual patient perspective, the utilization of the term NAFLD has led to many patients trivializing their condition. Several studies have reported that up to 95% of patients with suspected NAFLD are unaware of having liver disease, and that >75% do not feel they are at risk of developing NAFLD [34,35,37]. This minimization of potential harms does a disservice to the prevalence and potential severity of the disease, creating a lack of engagement among patient populations who suffer from NAFLD. Evidence suggests that trivialization mainly arises through an inappropriate name of the condition, or when disease perceptions or diagnoses are confusing to people. Expert opinion governing this area of terminology believe that the negative prefix “non-” carries a perception that the disease is unimportant [16].
Stigma associated with NAFLD diagnosis
One of the particularly onerous societal burdens of NAFLD is the utilization of alcohol in its name. Alcohol usage carries with it a significant stigma, and that stigma has overlapped into the diagnosis of NAFLD [16]. This is particularly damaging in discussing the disease with pediatric patients and practicing Muslim patients, where stigma may prohibit practitioners from discussing the disease with the patients. Recent correspondence regarding the change in terminologies’ impact on the Arab world, with the largest practicing Muslim population, has highlighted the benefit of changing the name to MAFLD [10,38].
Stigmatization of healthcare conditions carries a significant burden. Stigma has negative effects on self-esteem and can lead to decreased self-management of the condition, decreased quality of life, and increased inability to cope with a disease [16]. Stigma can also induce fear in patients, which can lead to adverse health behaviours, including denial of diagnosis, treatment avoidance, lack of compliance with treatment and healthcare advice, and ultimately, termination of treatment [16]. Therefore, stigma should be avoided with any diagnosis label to increase the patients’ motivation to manage their condition and to seek ongoing treatment.
Increase in prevalence of MAFLD compared with NAFLD
One of the benefits of utilizing MAFLD compared to NAFLD is the increase in the identification of individuals with high-risk features for progressive liver disease. In a study by Ayada et al. [39] (2021), 17 studies containing both a diagnosis of NAFLD and MAFLD comprising 9,808,677 individuals were reviewed. This study showed that the prevalence of MAFLD was 33.0% (95% CI 29.7–36.5), with a NAFLD prevalence of 29.1% (95% CI 27.1–31.1). The surprising detail of this study was that of all the fatty liver identified in the combined studies, 15.1% were identified with MAFLD-only diagnosis (95% CI 11.5–19.5). Several of the studies showed that large increases in the patients diagnosed were undertaken in Asian populations. This indicates that the new diagnostic criteria is better suited to identify patients over the traditional NAFLD diagnosis label [39]. Whilst this has been replicated in other reports, there are geographic variations to this increase in the identification of significant fatty liver disease.
Non-MR NAFLD
One of the potential detractions from utilizing MAFLD exists in the patients who fulfill the criteria for NAFLD without metabolic risk factors or other identifiable aetiologies of liver disease. When examining retrospective data comparing the two diagnoses, the majority of patients fulfill both the MAFLD and NAFLD criteria. However, there is a small proportion of patients who make up the non-MR NAFLD group across these studies, ranging from 0.6–16.1%, with most consistently estimating this group to make up around 5% of the fatty liver disease population [20,22,32,33,40-43]. While most risks were associated with MAFLD diagnosis, some studies did show that non-MR NAFLD patients had increased risks of cardiovascular disease during follow-up, though the majority of studies showed no increase in liver-related risk compared to the control populations [39]. The presence of severe hepatic steatosis has been shown to have implications on metabolic complications, including metabolic syndrome, and thus, these patients should be monitored for the development of complications, especially since metabolic risk factors, including weight and dysglycemia, can increase over time.
MAFLD CLINICAL DIFFERENCES
Mortality
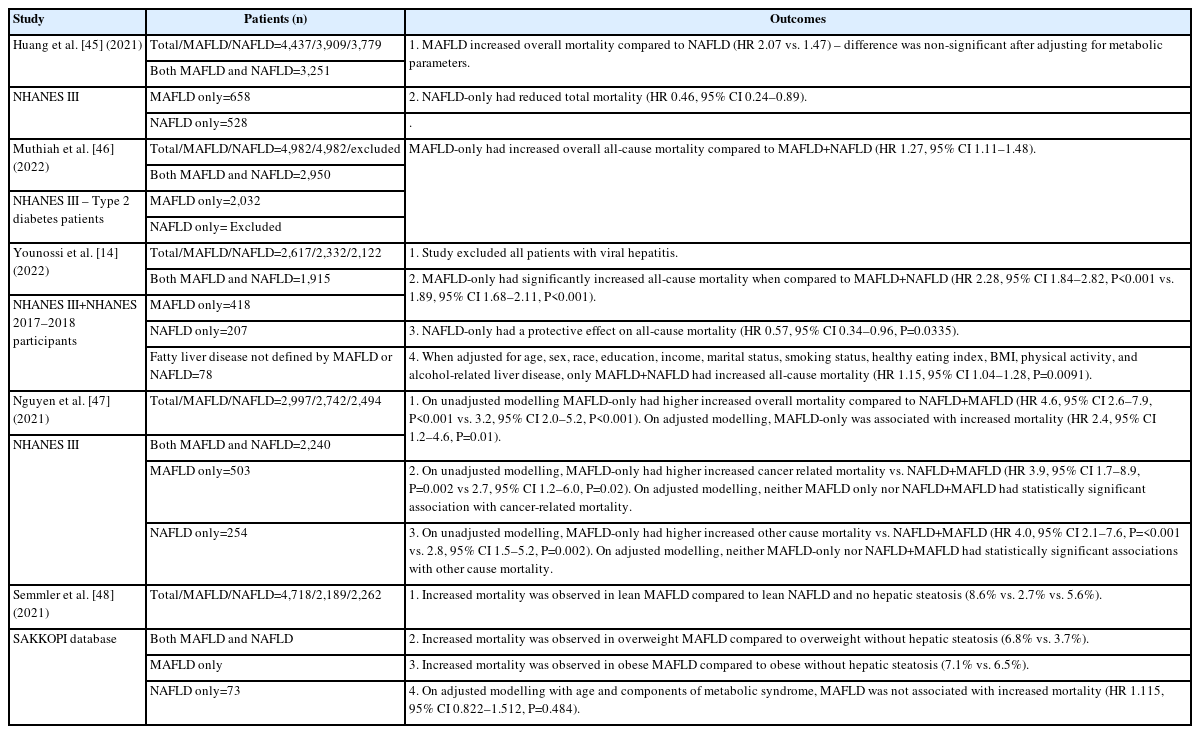
Whilst a number of articles have been published on MAFLD vs. NAFLD, there have been numerous negative articles suggesting that MAFLD does not contribute to mortality (Table 1) [14,44-48]. The main point suggested by these articles is that the metabolic dysregulatory features are the cause for mortality, and not the underlying MAFLD diagnosis. This has been shown using adjusted modelling considering type 2 diabetes mellitus and BMI, which were treated as confounders of the demonstrated association that MAFLD displays with mortality. However, there are two major concerns that these articles fail to acknowledge. First, as the diagnosis of MAFLD relies on metabolic dysregulation, type 2 diabetes mellitus and BMI cannot be treated as confounders—they are an integral part of the diagnosis. Put simply, without metabolic dysregulatory changes, there is no MAFLD; therefore, their inclusion as confounders in these adjustment models revokes the diagnosis of MAFLD. These adjustment models only assess fatty liver without a metabolic component, which is hepatic steatosis.
The second point that adjustment modelling indicates is that MAFLD without metabolic derangements does not have any association with mortality. While this has been highlighted to show that the MAFLD diagnosis is “wrong” and is then discussed at length, the opposite has been unwittingly demonstrated. What each of these articles has failed to recognize is that adjustment models show that the utilization of metabolic dysregulatory factors is the key cause of increased mortality in fatty liver deposition. Without further metabolic dysregulation, fatty liver per se poses no threat of increased mortality. As metabolic dysregulation is required for the diagnosis of MAFLD, in sum, these articles show that the consensus group was correct in selecting these factors to underpin the major causative pathways that lead to increased mortality.
A study by Moon et al. [44] (2022) assessed individuals from two community-based cohorts, between the ages of 40 and 70 years, and prospectively followed them for a median of 15.7 years. Using the diagnostic criterion for MAFLD and NAFLD and adjusting for confounders, they showed that MAFLD independently predicted the overall mortality with a hazard ratio (HR) 1.33 (95% CI 1.05–1.69), while NAFLD was not associated with the overall mortality with a HR of 1.20 (95% CI 0.94–1.53). MAFLD also predicted cardiovascular disease after adjustment for age, sex, and BMI, but lost its significance when adjusted for other metabolic dysfunction risk factors, most notably type 2 diabetes mellitus. The latter is not surprising, as discussed earlier, and since these risk factors are more proximal to adverse organ-specific outcomes (e.g., hypertension or atherogenic dyslipidemia for cardiovascular disease).
Metabolic risk factors
In utilization of the MAFLD criteria, there is an understanding of the individual phenotypic profiles of the patient that has contributed to the development of fatty liver infiltration [4]. These risks not only provide clues to the causation of fatty liver, but also on the possible treatment and management options. This is important when we address each of the individual phenotypes separately, but also when we note the synergistic effects that each pathway provides for the overall patient outcomes. In contrast, with the diagnosis of NAFLD, a one-size-f its-all approach governs the phenotypic presentation and management.
An example is the risk of type 2 diabetes mellitus in overweight or obese patients. It has been shown that being overweight or obese significantly increases the risks for developing type 2 diabetes mellitus [49]. The underlying mechanisms have not been fully established; however, weight loss in these individuals can ameliorate or even normalize the risk of type 2 diabetes mellitus. Targeted weight loss should be the first step in reducing the risk of developing type 2 diabetes mellitus in overweight or obese patients by decreasing peripheral and hepatic insulin resistance [49]; whereas, in patients with type 2 diabetes mellitus, the first step in management is the normalization of blood sugars. Whilst this can be targeted by weight loss, the specific goal is the normalization of blood sugars. This highlights the contributory metabolic risk factors in the development of further metabolic co-morbidities. Each needs a tailored response to address the underlying needs, despite the interrelated effects of each.
Of note, there have been recent studies demonstrating that a MAFLD diagnosis is associated, on multivariate analysis, with an increased risk of type 2 diabetes mellitus in patients whose metabolic phenotype at diagnosis does not include type 2 diabetes mellitus, compared to a NAFLD diagnosis [50]. This is significant as it demonstrates the early diagnosis of MAFLD over that of NAFLD, which can help target individuals at risk of developing other significant complications, such as type 2 diabetes mellitus. It, therefore, allows clinicians to appropriately target patient populations to modify their metabolic risk profile to prevent complications [50].
Utilizing the definition of MAFLD also highlights the importance of holistic patient management [4]. Currently, the mainstay of initial management of all metabolic disorders is dietary change and exercise. Targeting them holistically, rather than in an organ-specific manner, can lead to widespread improvements in outcomes, particularly with regard to cardiovascular health and cancer, which are the greatest causes of adverse outcomes in fatty liver disease [5]. This is also particularly important for clinical research which focuses on metabolic dysregulation to improve both liver and systemic outcomes.
Metabolic complications
Outside of the traditional metabolic dysregulatory environments that are included in the diagnostic algorithm for MAFLD, there have been studies that showed an association between MAFLD and other disease processes [51,52].
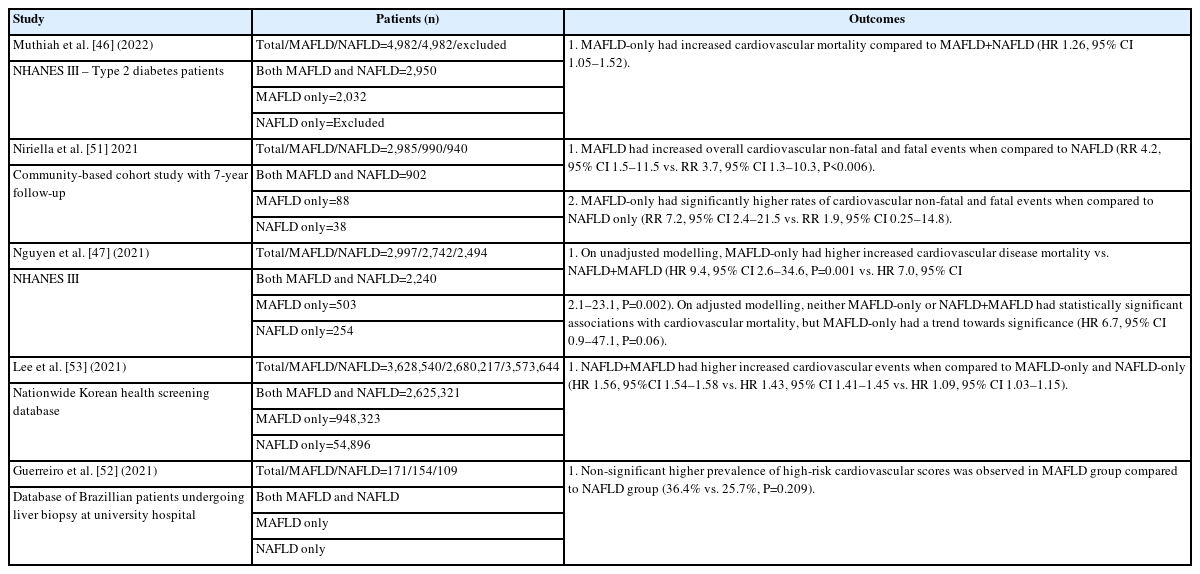
This is to be expected when placing MAFLD in alignment with other metabolic dysregulation-associated disorders, such as cardiovascular disease, rather than the stand-alone disease entity of NAFLD (Table 2). While cardiovascular disease is the major mortality burden in fatty liver disease, other disorders associated with MAFLD include peripheral vascular disease, chronic kidney disease, and some cancers, especially of the gastrointestinal tract [5].
There have been studies assessing the cardiovascular risk association of MAFLD vs. NAFLD. A study by Lee et al. [53] (2021) evaluated incident cardiovascular disease risk from a nationwide health screening database involving 9,584,399 participants followed for a median of 10.1 years. Patients were placed in fatty liver disease (FLD), NAFLD-only, MAFLD-only, or both FLD groups. Cardiovascular risk was elevated in all fatty liver disease; however, NAFLD-only group had significantly decreased hazard ratio (HR 1.09, 95% CI 1.03–1.15) compared to MAFLD-only (HR 1.43, 95% CI 1.41–1.43) and both FLD groups (HR 1.56, 95% CI 1.54–1.58).
Recent studies assessing NAFLD vs. MAFLD have identified that asymptomatic atherosclerotic cardiovascular disease has an independent association on multivariable logistic regression models with MAFLD, but not with NAFLD diagnosis [54]. This is significant due to the burden of cardiovascular disease in patients suffering from fatty liver infiltration. Therefore, MAFLD diagnosis assists in identifying patients who should undergo cardiovascular assessment and intervention over the traditional NAFLD diagnosis.
Non-metabolic complications
Other associations made with NAFLD have been assessed against the MAFLD criteria to assess the strength of the associations with the change in terminology. A study by Sun et al. [49] (2021) utilized the NHANES database to assess the correlation of MAFLD with chronic kidney disease (CKD) and abnormal albuminuria. In that study, MAFLD patients had a lower estimated glomerular filtration rate (74.96±18.21 vs. 76.46±18.24 mL/min/1.73m2, P<0.001) and a greater prevalence of CKD (29.60% vs. 26.56%, P<0.005) compared to those with NAFLD.
Studies addressing the association between MAFLD and other conditions are currently underway. While several conditions, such as breast lesions, have shown that MAFLD is related with these conditions, similar to NAFLD, no direct comparison has been published. It would be interesting to note the strength of association of the conditions that were previously noted to be associated with NAFLD, as well as the impact of the MAFLD criteria on them.
Somewhat surprisingly, MAFLD has shown associations with lung conditions over a NAFLD diagnosis, with poorer lung function and higher rates of mortality associated with COVID19 infection. A study performed by Miao et al. [56] (2022) compared the association of lung function parameters in patients diagnosed with MAFLD vs. NAFLD. After adjusting for age, sex, adiposity measures, smoking status, and alcohol intake, MAFLD subjects had significantly lower predicted forced vital capacity (88.27±17.60% vs. 90.82±16.85%, P<0.005) and lower 1 second forced expiratory volume (FEV1) (79.89±17.34 vs. 83.02±16.66%, P<0.005) when compared to those diagnosed with NAFLD. While the results suggest that MAFLD has a greater role in identifying patients with reduced lung function, it is likely related to MAFLD selecting patients with higher non-invasive liver fibrosis scores. Every 1-point increase in FIB-4 resulted in a decrease in FVC by 0.507 (95% CI –0.840 to –0.173, P=0.003) and a decrease in FEV1 by 0.439 (95% CI –0.739 to –0.140, P=0.004).
Dual etiology liver disease and synergistic effects
The additive basis of MAFLD with other liver diseases is a main advantage over the traditional NAFLD definition. Since NAFLD excludes concomitant liver diseases, such as hepatitis B or C, there was no ability for the patients to have dual etiologies for their liver disease. Substantive literature has shown that individuals who have underlying liver diseases from hepatitis B and hepatitis C, with a diagnosis of MAFLD, have significantly increased complications, both intra- and extra-hepatic [13]. The additional diagnosis of MAFLD coupled with hepatitis B, for example, increases the rates of complications and mortality [57].
In a recent study by Zheng et al. [58], among 780 patients with liver biopsies, 773 were given a diagnosis of MAFLD. Of the patients with MAFLD, 66 also had excess alcohol consumption. On subgroup analysis assessing MAFLD patients with significant alcohol consumption, the patients had high gamma-glutamyl transferase levels and exhibited more hepatic steatosis when compared to patients with MAFLD without co-existing liver disease. This outcome could not be evaluated in previous studies with NAFLD due to the requirement to exclude co-existing liver disease.
Future treatment pathways – Exclusionary diagnosis of NAFLD limits treatment options for patients
Due to the restrictive nature of NAFLD not allowing concurrent liver disease as a requirement for diagnosis, treatment strategies have focused on single liver disease entities [11]. With the more finessed MAFLD diagnosis, the co-existence of separate entities of liver disease can be entertained [4]. This allows clinicians to manage one or more conditions simultaneously, rather than treating a “dominant” liver disease.
While there is currently no approved medical treatment for MAFLD, there are a number of phase III trials underway that are showing promising preliminary results [59]. One of the major benefits of a MAFLD diagnosis, which has been overlooked in the debate over terminology, is the potential inability to provide treatment for fatty liver infiltration in individuals with concurrent liver pathologies [4,7]. This underscores the most serious implication of the NAFLD terminology in excluding significant proportions of the population who would benefit from future treatments.
MAFLD RESEARCH
Exploring phenotypic conditions
The inclusion for NAFLD clinical studies has been based on a hepatic phenotype in the absence of significant alcohol intake and all concurrent steatosis-associated liver pathologies. The move forward with MAFLD proposes that the basis for intervention should focus on the pathogenic drivers. This change will move research on fatty liver from a “one-size-fits-all” situation to a more nuanced treatment of its pathophysiological determinants [36].
Previous correspondence has suggested that the name change to MAFLD may hinder the interpretation of studies that are currently ongoing [60]. The major concern is regarding the utilization of “resolution of NASH with no worsening of liver fibrosis,” which is a key histological endpoint for conditional drug approval [61]. Negating this argument, the MAFLD criteria do not propose any change in pathological criteria for a diagnosis of metabolic steatohepatitis.
Positive diagnostic criteria – Less confounding bias in patient selection for research
When selecting patients for fatty liver disease trials, there is confounding bias associated with the NAFLD terminology [62]. Whilst the exclusionary criteria of alcohol and other contributory liver diseases are standard, there is no mechanism to explore the pathogenic aspects of the underlying liver fat infiltration. We have already discussed concerns with alcohol usage in patients with NAFLD with significant underestimation likely in clinical practice. With the utilization of MAFLD and the strict criteria for assessing metabolic co-factors, however, clinical trials inclusion will identify a more homogenous group of patients.
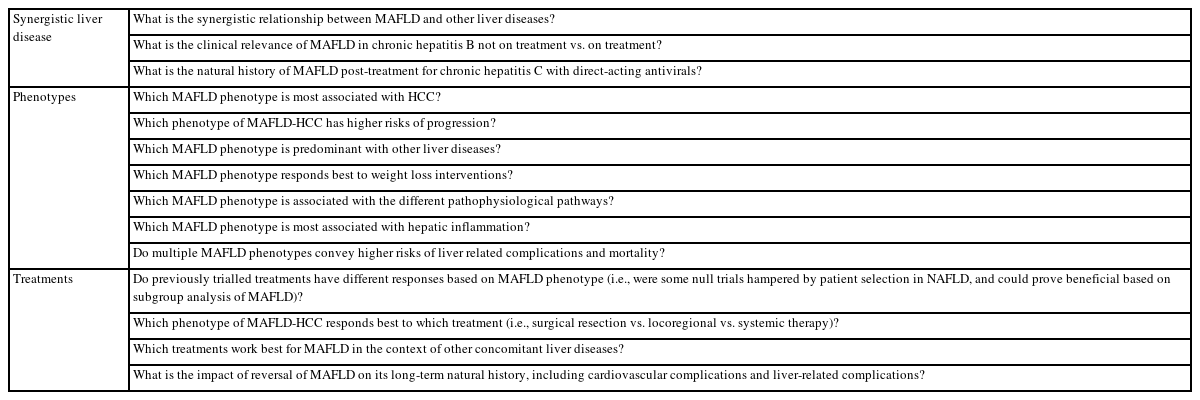
While the controversy regarding NAFLD vs. MAFLD is ongoing, the debate is also polarizing. Although MAFLD will not capture every single patient, it does capture those who require early intervention and are at increased risk of disease progression. Therefore, in our perspective, it would on balance be more beneficial to further develop the MAFLD concept for improved patient care and clinical research (Table 3).
CONCLUSION
There are significant clinical, research, and patient benefits to the utilization of MAFLD over the NAFLD terminology. MAFLD establishes a clear diagnosis due to a set of positive diagnostic criteria that allows clinicians to better tailor practice to target individuals at high risk of developing complications or other metabolic co-morbidities. Therefore, we contend that the term “MAFLD” is a step in the right direction to decrease the stigma associated with a NAFLD diagnosis, to increase public awareness and to improve clinical care.
Notes
Authors’ contribution
All authors were responsible for drafting and critical revision of the manuscript.
Conflicts of Interest
The authors have no conflicts to disclose.
Abbreviations
MAFLD
metabolic dysfunction-associated fatty liver disease
NAFLD
nonalcoholic fatty liver disease
NASH
non-alcoholic steatohepatitis
ALRD
alcohol-related liver disease
BMI
body mass index
NHANES
National Health and Nutrition Examination Surveys
FIB-4
fibrosis-4
FLD
fatty liver disease
CKD
chronic kidney disease
FEV1
forced expiratory volume in 1 second