Association of non-alcoholic fatty liver disease with incident dementia later in life among elder adults
Article information
Abstract
Background/Aims
Accumulating evidence suggests a link between non-alcoholic fatty liver disease (NAFLD) and brain health. However, population-based evidence on the association between NAFLD and dementia remains unclear. This study was conducted to determine the association between NAFLD and incident dementia.
Methods
The study population included 608,994 adults aged ≥60 years who underwent health examinations between 2009 and 2010. Data were collected from the Korean National Health Insurance Service database. NAFLD was assessed using the fatty liver index (FLI). A Cox proportional hazards regression model was used to determine the association between NAFLD and dementia.
Results
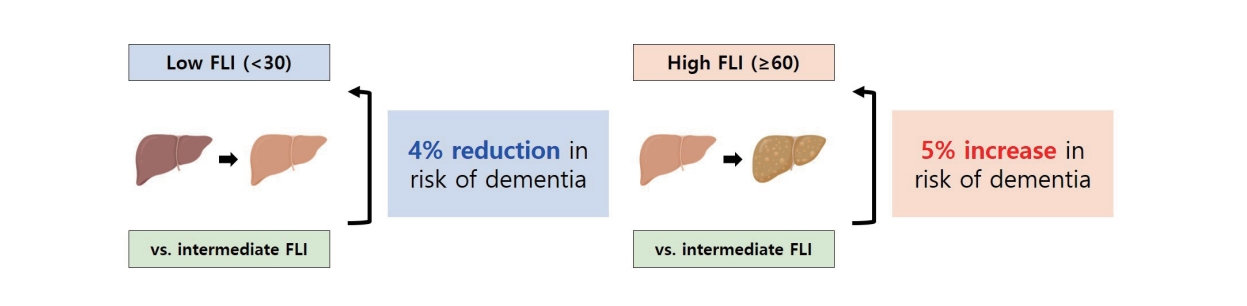
During the 6,495,352 person-years of follow-up, 48,538 participants (8.0%) developed incident dementia. The participants were classified into low (FLI <30), intermediate (FLI ≥30 and <60), and high (FLI ≥60) groups. In the overall study population, the FLI groups were associated with a risk of dementia (P for trend <0.001). After propensity score matching, a low FLI was associated with a reduced risk of dementia (adjusted hazard ration [aHR], 0.96; 95% confidence interval [CI], 0.93–0.98; P=0.002), whereas a high FLI (NAFLD) was associated with an increased risk of dementia (aHR, 1.05; 95% CI, 1.02–1.08; P=0.001). A higher risk of dementia in the high FLI group than in the intermediate FLI group was attributed to Alzheimer’s disease (aHR, 1.04; 95% CI, 1.01–1.07; P=0.004) rather than vascular dementia (aHR, 0.94; 95% CI, 0.75–1.18; P=0.602).
Conclusions
NAFLD was associated with an increased risk of dementia, which was attributed to an increased risk of Alzheimer’s disease.
Graphical Abstract
INTRODUCTION
Approximately 50 million people suffer from dementia worldwide, and the number of elderly individuals with dementia continues to increase in the context of global population aging, placing a significant burden on the healthcare system [1-3]. Non-alcoholic fatty liver disease (NAFLD) is also increasing in prevalence as a representative non-communicable disease of the liver, affecting up to a quarter of the adult population in parallel with a global epidemic of obesity and metabolic syndrome [4]. Minimizing exposure to modifiable risk factors for dementia has been reported to reduce the incidence of dementia in several cohort studies [5-7]. In particular, a population-based study suggested that regular physical activity and management of cardiovascular risk factors may reduce the risk of cognitive decline and dementia [8]. Likewise, the elucidation and management of risk factors are important in reducing the incidence and burden of dementia.
Recent studies have yielded inconsistent results regarding the relationship between NAFLD and dementia. According to a previous National Health and Nutrition Examination Survey study, NAFLD was associated with cognitive impairment in the general USA population, independent of cardiovascular disease and its risk factors [9]. In contrast, a German cohort study demonstrated that neither the incidence of overall dementia, nor that of vascular dementia, was associated with NAFLD [10]. According to a Swedish cohort study, NAFLD itself was not associated with incident dementia; however, liver histology, especially fibrosis stage, could improve the predictive performance of dementia risk [11]. In addition, the Framingham study suggested that the presence of NAFLD was not associated with cognitive function, but the NAFLD fibrosis score (NFS) could predict cognitive impairment in patients with NAFLD [12]. Conversely, an Italian study demonstrated that NFS was not a significant risk factor for dementia [13]. Given the contradictory results, further larger-scale population-based studies that explore the potential impact of NAFLD on the risk of dementia are warranted. This study investigated the association of NAFLD with the risk of incident dementia, including Alzheimer’s disease and vascular dementia, based on the fatty liver index (FLI).
PATIENTS AND METHODS
Study population
Detailed information regarding the validity and design of the Korean National Health Insurance Service (NHIS) is described in a previous study [14]. Briefly, the NHIS is an insurance system established under the Ministry of Health and Welfare, which covers approximately 97% of the Korean population. The NHIS collects demographic characteristics, health screening results, healthcare and treatment, drug prescription, and questionnaire-based behavioral characteristics, and carries out quality control before providing data for research purposes.
This study used data from the nationwide Korean NHIS database. There were 3,269,657 older adults aged ≥60 years who underwent health examinations between 2009 and 2010. Participants with a history of ischemic heart disease (International Classification of Diseases tenth revision [ICD-10], I20-I25; n=321,377), arterial hypertension (ICD-10, I10; n=970,856), heart failure (ICD-10, I50; n=45,753), renal failure (ICD-10, N18 and N19; n=3,221), stroke and transient ischemic attack (ICD-10, I60-I64 and G45; n=79,228), intracranial injury (ICD-10, S06; n=23,224), epilepsy (ICD-10, G40 and G41; n=6,345), Parkinson’s disease (ICD-10, G20 and G21; n=4,990), osteoporosis (ICD-10, M80 and M81; n=218,147), and depression (ICD-10, F32 and F33; n=33,848), before the follow-up investigation of dementia, were excluded. In addition, those with missing information on alcohol consumption (n=12,303) and those with alcohol consumption ≥1 times/week (n=727,822) were excluded. Among the remaining non-drinking older adults (n=822,543), participants with a history of dementia (n=60,353) prior to the follow-up investigation were also excluded from analysis. In addition, participants with missing information on the evaluation of the FLI, adjustment analysis, and stratified analysis, and those with chronic viral hepatitis infection (ICD-10, B18; n=102,146), autoimmune hepatitis (ICD-10, K754; n=89), primary biliary cholangitis (ICD-10, K743; n=60), and primary sclerosing cholangitis (ICD-10, K830; n=541) before the follow-up investigation were excluded from the analysis. None of the participants had Wilson’s disease (ICD-10, E8301) or hemochromatosis (ICD-10, E8311) before enrollment. The final analytic cohort consisted of 608,994 participants (Fig. 1). This study was conducted in accordance with the Declaration of Helsinki and the STROBE guidelines. The Institutional Review Board of Seoul National University approved this study (E-1803-045-928). The requirement for informed consent was waived because the NHIS database provided anonymized data in accordance with the Personal Data Protection Act guidelines.
Follow-up for incident dementia
In the present study, dementia was operationally defined based on the ICD-10 codes F00, F01, F02, F03, and G30, along with dementia-associated medication use, including donepezil, galantamine, rivastigmine, and memantine. Alzheimer’s disease was diagnosed when a participant had ICD-10 codes F00 and G30, whereas vascular dementia was diagnosed using the ICD-10 code F01 on the basis of the use of dementia-associated medications. All participants were followed from the date of health examination to the date of incident dementia, death, or December 31, 2020.
Evaluation of fatty liver and metabolic syndrome
NAFLD was defined using the FLI, which was calculated using the following formula:
Low and high FLIs were defined using the dual cutoffs of FLI (<30 and ≥60, respectively). The FLI is considered an acceptable alternative to imaging modalities according to the European Clinical Practice Guidelines [16]. In the Korean population, the FLI was validated with an area under the curve value of 0.87 in a receiver operating characteristic curve [17].
The National Cholesterol Education Program Adult Treatment Panel III was adopted to define metabolic syndrome as when three or more of the following criteria were met: waist circumference ≥90 cm for men and ≥80 cm for women, systolic blood pressure ≥130 or diastolic blood pressure ≥85 mmHg, triglyceride level ≥150 mg/dL, high-density lipoprotein cholesterol ≤40 mg/dL for men or ≤50 mg/dL for women, and fasting serum glucose (FSG) ≥100 mg/dL [18].
Key variables
The following covariates were considered key variables for multivariate analyses: age (continuous; years), sex (categorical; men and women), household income (categorical; upper half and lower half), BMI (continuous; kg/m2), systolic blood pressure (continuous; mmHg), FSG (continuous; mg/dL), smoking (categorical; never, previous, and current), moderate-to-vigorous physical activity (categorical; ≤2, 3–4, and ≥5 times/week), and Charlson comorbidity index (CCI; continuous). CCI was calculated as described in a previous study [19].
Statistical analysis
Categorical and continuous variables are presented as number (%) and median (interquartile range [IQR]), respectively. The Cox proportional hazards model was adopted to evaluate adjusted hazard ratios (aHRs) with 95% confidence intervals (CIs). The following models were analyzed to estimate the risk of incident dementia: model A, adjusted for age, sex, and BMI; model B, adjusted for age, sex, BMI, household income, systolic blood pressure, and FSG; and model C, adjusted for smoking, moderate-to-vigorous physical activity, and CCI in addition to factors included in model B.
Among the key variables, only independent predictive factors for dementia that were significant in the multivariate Cox regression analysis were selected as covariates for propensity score matching (PSM) to reduce confounding effects. PSM was conducted against the intermediate FLI group for both low and high FLI groups. A caliper of width equal to 0.2 of the standard deviation of the logit of the propensity score was used for 1:1 matching of subjects between the different FLI groups. The number of participants after PSM was 144,299 for low FLI and 145,799 for high FLI. The matched proportions of participants with low and high FLI were 75.0% and 54.2%, respectively.
Sensitivity analyses were performed after washing out the selected latent periods by excluding participants with dementia within the defined selected periods. Age, sex, BMI, hypertension, diabetes mellitus, dyslipidemia, smoking, physical activity, CCI, and metabolic syndrome were considered for stratified analyses to evaluate the interaction with FLI. The supremum test was performed to test the proportional hazards assumption in the Cox model. Statistical significance was set at P<0.05. All statistical analyses were performed using SAS Enterprise Guide 7.1 (SAS Institute, Cary, NC, USA).
RESULTS
Participant characteristics
Table 1 presents the baseline characteristics of the study participants in the Korean NHIS cohort. Study participants included 192,874 men (31.6%) and 416,810 women (68.4%) with a median age of 65 years (IQR, 62–69). More than half of the participants (n=374,765; 61.5%) belonged to the upper half of their household income. The median BMI and waist circumference were 23.6 kg/m2 and 81 cm, respectively. The majority of the participants had never smoked (n=533,722; 87.5%). In addition, hypertension, diabetes mellitus, and dyslipidemia were present in 82,603 (13.5%), 52,594 (8.6%), and 145,015 (23.8%) of participants, respectively. According to the FLI category, the participants were classified into the low (FLI <30; fatty liver rule-out; n=193,897), intermediate (FLI ≥30 and <60; n=145,814), and high (FLI ≥60; fatty liver rule-in; n=269,441) groups. The participants in the high FLI group were more likely to be men with higher levels of BMI, waist circumference, blood pressure, total cholesterol, and FSG.
Association of NAFLD with incident dementia
During the 6,495,352 person-years of follow-up, 48,538 participants (8.0%) developed incident dementia. In addition, 46,852 (7.7%) and 688 (0.1%) participants developed Alzheimer’s disease and vascular dementia, respectively (Table 2). In the fully adjusted model (model C), the risk of dementia was associated with FLI groups (P for trend <0.001), and a low FLI was associated with a decreased dementia risk (aHR, 0.97; 95% CI, 0.94–0.99) compared to the intermediate group. Alzheimer’s disease was significantly associated with the FLI group (P for trend=0.004), whereas vascular dementia was not associated with the FLI group (P for trend=0.117). Sensitivity analyses, after excluding latent periods for the development of dementia, demonstrated similar results to the primary findings (Supplementary Table 1).
Association of NAFLD with incident dementia in the PSM cohort
We sought to determine whether a high FLI was associated with an increased dementia risk after PSM. Multivariate Cox regression analysis of the key variables included in the adjustments identified age, sex, BMI, household income, systolic blood pressure, FSG, smoking, moderate-to-vigorous physical activity, and CCI as independent factors associated with dementia risk (Supplementary Table 2). The logistic regression analysis results for PSM are shown in Supplementary Table 3. The descriptive statistics of the participants after PSM for the intermediate-and low-FLI groups are presented in Supplementary Table 4. Descriptive characteristics after PSM of subjects with a high FLI versus those with an intermediate FLI are shown in Supplementary Table 5.
After PSM, a low FLI was associated with a lower dementia risk (aHR, 0.96; 95% CI, 0.93–0.98; P=0.002) in the final adjustment model (Table 3). In addition, a low FLI was associated with a lower risk of Alzheimer’s disease (aHR, 0.96; 95% CI, 0.93–0.99; P=0.005) but not with vascular dementia (aHR, 0.84; 95% CI, 0.66–1.07; P=0.157) in the final adjustment model. In the matched cohort of both the intermediate and high FLI groups, a high FLI was associated with a higher dementia risk (aHR, 1.05; 95% CI, 1.02–1.08; P=0.001). A significant association between high FLI and higher dementia risk was attributed to the increased risk of Alzheimer’s disease in the high FLI group (aHR, 1.04; 95% CI, 1.01–1.07; P=0.004) but not to vascular dementia (aHR, 0.94; 95% CI, 0.75–1.18; P=0.602) in the final adjustment model.
Stratified analysis of low versus intermediate FLI groups on the risk of incident dementia
After stratification of participants, a significant interaction was found between sex, hypertension, and dyslipidemia (Supplementary Table 6). A low FLI was associated with a decreased dementia risk in any age, sex, BMI <25 kg/m2, hypertension, no diabetes mellitus, no dyslipidemia, never smoking, and moderate-to-vigorous physical activity ≤2 times/ week. According to metabolic health, a low FLI showed a lower dementia risk in participants without metabolic syndrome and normal waist circumference, blood pressure, high-density lipoprotein cholesterol, and FSG.
Stratified analysis of high versus intermediate FLI groups on the risk of incident dementia
Stratified analyses of the high-and intermediate-FLI groups are shown in Supplementary Table 7. No significant interactions were found between the selected variables used for stratification. A high FLI was associated with a higher risk of dementia among older adults, women, both BMI, no hypertension, no type 2 diabetes, no dyslipidemia, never and current smokers, moderate-to-vigorous physical activity ≤2 times/week and ≥5 times/week, CCI=1, no metabolic syndrome, normal waist circumference, abnormal blood pressure, normal triglyceride, normal high-density lipoprotein cholesterol, and normal FSG subgroups, as compared to an intermediate FLI.
DISCUSSION
The global epidemic of obesity has fueled the rapidly increasing burden of NAFLD, which has become a leading cause of end-stage liver diseases, hepatocellular carcinoma, and cardiometabolic diseases [20]. In the present study, FLI as a proxy for NAFLD was significantly associated with the risk of incident dementia. A significant association between high FLI and overall incident dementia attributable to Alzheimer’s disease was found after PSM. Therefore, the management of NAFLD may reduce the disease burden related to dementia. In addition, exploring the underlying mechanisms linking NAFLD to incident dementia may provide new insights into preventive and therapeutic strategies against the development and progression of dementia.
Weinstein et al. [21] examined the relationship between NAFLD and total brain volume in 906 subjects enrolled in the Framingham offspring cohort. There were no significant associations between white matter hyperintensities and hippocampal volume, but they found a significant association with total brain volume. Even after adjustment for the covariates, patients with NAFLD had smaller-than-normal brains for their age, which can be seen as a pathologic acceleration of the brain aging process. This finding was most striking among the youngest subjects, accounting for about a 7-year advance in brain aging for those younger than 60 years. Taken together, the contribution of fatty liver to dementia risk may be due to its biological effect on brain aging.
A growing body of research has linked insulin resistance to several neurodegenerative mechanisms of Alzheimer’s disease, including oxidative stress, mitochondrial dysfunction, and chronic liver inflammation, via dysregulated insulin/insulin-like growth factor 1 signaling with accompanying impairments in signal transduction and gene expression [22-24]. A network clustering analysis demonstrated that 189 genes were shared between Alzheimer’s disease and NAFLD [25]. The identified main pathways contributing to both Alzheimer’s disease and NAFLD included carbohydrate metabolism, fatty acid metabolism, and interleukin-17 signaling pathways.
NAFLD may also increase amyloid burden and aggravate Alzheimer’s pathology. This contribution can be largely attributed to an imbalance in peripheral amyloid-β (Aβ) clearance as a result of a reduction in low-density lipoprotein receptor-related protein 1 (LRP-1) levels that are highly expressed in hepatocytes under physiological conditions [26]. Liver dysfunction is accompanied by low expression of hepatic LRP-1 and high levels of circulating Aβ, suggesting that Aβ clearance decreases due to low hepatic LRP-1 expression. Alternatively, insulin promotes LRP-1 translocation to the cell membrane in hepatocytes, favoring Aβ clearance [27]. The stimulation of LRP-1-mediated liver uptake indeed ameliorates cognitive dysfunction and decreases Aβ aggregation in the brains of Alzheimer’s disease transgenic mice [28]. These features may also disrupt the blood-brain barrier and contribute to a vicious cycle.
Alzheimer’s disease is an irreversible neurodegenerative disease in which neuroinflammation plays a critical role [29]. A preclinical study demonstrated that NAFLD-induced chronic liver inflammation contributes to the pathogenesis of Alzheimer’s disease by inducing neurodegeneration in a genetic predisposition-absent setting [24]. They showed that NAFLD induced by a high-fat diet (HFD) promotes the development of Alzheimer’s disease in mice. Brains of HFD-fed mice revealed increased levels of neuroinflammation with higher levels of pro-inflammatory cytokines, toll-like receptors, and microgliosis, which were accompanied by increased plaque formation in Alzheimer’s disease transgenic mice. Furthermore, lipocalin-2 (Lcn2) is an adipokine exclusively produced in the liver and circulates throughout the body among individuals with nonalcoholic steatohepatitis (NASH) [30]. Recently, a murine model of NASH revealed that high levels of Lcn2 circulating in the bloodstream can activate a number of pro-inflammatory processes in the brain. The study also suggested that Lcn2 induces a weakening of the blood-brain barrier, which subsequently increases the expression of inflammatory molecules in brain endothelial cells [31].
The adaptive immune response has been found to contribute to the development of Alzheimer’s disease [32]. Adaptive immune responses were noticeable in the blood and cerebrospinal fluid collected from patients with Alzheimer’s disease, with clonal antigen-experienced CD8+ T cells patrolling the intrathecal space of the brain and are affected by age-associated neurodegeneration [33]. The evolution of NAFLD to NASH is accompanied by an increased frequency of intrahepatic cytotoxic CD8+ T cells [34]. These cells were recruited in response to signals modulated by interferon-α, and exacerbated insulin resistance and glucose intolerance in the livers of HFD-fed mice [35]. In addition, mice lacking CD8+ T cells and natural killer T (NKT) cells were protected from steatosis when fed a choline-deficient HFD, which was related to a reduction in soluble mediators, such as lymphotoxin-like inducible protein that competes with glycoprotein D for binding herpes virus entry mediator on T cells and lymphotoxin, released by CD8+ T cells and NKT cells [36]. Furthermore, the selective ablation of CD8+ T cells demonstrated effectiveness in the amelioration of steatohepatitis in mice fed a high-fat and high-carbohydrate diet, indicating a pathogenic role of adaptive immunity in the development of NASH [37]. These findings suggest a close relationship between intrahepatic adaptive immunity and adaptive immune response within the brain, which awaits further experimental validation.
Recent studies have suggested that advanced fibrosis may impact the risk of cognitive dysfunction and incident dementia [11,12]. Although the exact pathogenic mechanism for cognitive impairment in individuals with NASH and advanced fibrosis remains unclear, neuroinflammation and changes in brain-derived neurotrophic factor levels may interact on the same causal pathway of liver fibrosis and cognitive dysfunction [38-40]. We speculate that liver fibrosis may result in the overexpression of pro-inflammatory cytokines, leading to a reduction in brain-derived neurotrophic factor levels, and ultimately to cognitive impairment. Further studies are required to define the association between advanced fibrosis and the risk of dementia. Stratified analyses revealed that current smokers with low FLI had no significant beneficial effects on dementia risk compared to those with intermediate FLI, but high FLI subjects showed a significant increase in dementia risk. In addition, dementia risk was significantly reduced only in participants with a lower BMI. These results suggest that modification of lifestyle behaviors (i.e., smoking cessation and weight loss) should be accompanied in the evaluation of NAFLD-associated dementia risk.
This study had some limitations that need to be considered. First, NAFLD was operationally defined using the FLI. Further larger-scale validation based on radiologic or pathologic confirmation of NAFLD may strengthen the intrinsic association between fatty liver and dementia. However, FLI evaluation allows the identification of the low FLI group, which is difficult to implement using conventional approaches in a real-world setting. Second, our study population consisted only of an East Asian population. Considering ethnicity-related differences in BMI and waist circumference, our results require further validation in other ethnic populations. Third, although additional functional studies based on gene expression and biological characteristics were not conducted in the present study, our findings merit further mechanistic investigation. In addition, despite the exclusion of participants with alcohol consumption with a frequency of ≥1 times/week, we might have failed to identify those with heavy alcohol consumption at an undetectable frequency (<1 time/week). Lastly, not all potential covariates that may be associated with dementia risk, such as education level, could be included in the adjustment. Nevertheless, our study is the first large-scale population-based study to explore the association of fatty liver with incident dementia at a nationwide level.
In conclusion, NAFLD, defined using the FLI, is independently associated with a higher risk of incident dementia attributable to Alzheimer’s disease, whereas a low FLI was associated with a lower risk of dementia. Although additional research is warranted to further clarify the underlying mechanism, accumulating evidence of the link between fatty liver and brain health, such as an epidemiologic association, may be mediated by the complex interplay between metabolism and vascular function in the liver.
Notes
Authors’ contributions
Conceptualization: SJ, YHO, SC, JC, SMK, JSS, GL, JCA, DHL, BKK, WK, and SMP. Data curation: SJ, SC, JC, SMK, and SMP. Formal analysis: SJ, SC, JC, and SMK. Methodology: SJ, SC, JC, SMK, JCA, DHL, BKK, WK, and SMP. Supervision: WK and SMP. Writing – original draft: SJ, YHO, SC, WK, and SMP. Writing – review & editing: SJ, YHO, SC, JSS, GL, JCA, DHL, BKK, WK, and SMP.
Conflicts of Interest
The authors have no conflicts to disclose.
Acknowledgements
Won Kim received a National Research Foundation of Korea (NRF) grant funded by the Korean Government (NRF2021R1A2C2005820 and NRF-2021M3A9E4021818). Dong Hyeon Lee received the grant from the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (HI21C0538). Sang Min Park received the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT; Grant number: 2021R1F1A1063346) and the SNUH Research Fund (04-2021-0370).
SUPPLEMENTAL MATERIAL
Supplementary material is available at Clinical and Molecular Hepatology website (http://www.e-cmh.org).
Sensitivity analyses on the risk of incident dementia according to the FLI category
Multivariate analysis of the key variables on the risk of dementia
Multivariate logistic regression of independent significant factors affecting the risk of dementia for propensity score matching
Descriptive statistics of the participants with intermediate and low FLI after propensity score matching
Descriptive statistics of the participants with intermediate and high FLI after propensity score matching
Subgroup analyses on the risk of incident dementia according to the FLI category in the propensity score matching cohort
Subgroup analyses on the risk of incident dementia according to the FLI category in the propensity score matching cohort
Abbreviations
aHR
adjusted hazard ratio
Aβ
amyloid-β
BMI
body mass index
CCI
Charlson comorbidity index
CI
confidence interval
FLI
fatty liver index
FSG
fasting serum glucose
HFD
high-fat diet
ICD-10
International Classification of Diseases tenth revision
IQR
interquartile range
Lcn2
lipocalin-2
LRP-1
lipoprotein receptor-related protein 1
NAFLD
non-alcoholic fatty liver disease
NASH
nonalcoholic steatohepatitis
NFS
NAFLD fibrosis score
NHIS
National Health Insurance Service
NKT
natural killer T
PSM
propensity score matching
References
Article information Continued
Notes
Study Highlights
• A significant association between nonalcoholic fatty liver disease and dementia
• Participants with higher fatty liver index had a higher risk of dementia.
• Management of fatty liver may lower the risk of dementia in elder adults.