Statin and aspirin for chemoprevention of hepatocellular carcinoma: Time to use or wait further?
Article information
Abstract
Preclinical studies highlighted potential therapeutic applications of aspirin and statins as anticancer agents based on their pleiotropic effects. Epidemiologic studies suggested the role of aspirin and statins in the chemoprevention of hepatocellular carcinoma (HCC). However, observational data is prone to bias, and no prospective randomized trials are currently available to assess the risks and benefits of statin or aspirin therapy for chemoprevention of HCC. It is therefore important for clinicians and researchers to be aware of the quality of current evidence regarding this issue. In this review, we summarize currently available evidence to assist clinicians with their decision to use statin or aspirin and provide information for further clinical investigations.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related mortality worldwide, with growing incidence and mortality in both Western and Asian countries [1,2]. HCC typically occurs in patients with chronic infections with hepatitis B virus (HBV) and hepatitis C virus (HCV), heavy alcohol intake and nonalcoholic fatty liver disease (NAFLD) [3,4]. The use of antiviral agents, such as nucleos(t)ide analogs (NAs) or direct-acting antivirals, has significantly decreased the complications of viral hepatitis-associated liver disease; however, it does not completely eliminate the risk of HCC in high-risk patients, including those with advanced fibrosis [5]. Therefore, an effective strategy focused on preventing the development of HCC in at-risk population remains a clinical unmet need.
Recent experimental and epidemiological studies have highlighted the potential therapeutic applications of aspirin and statins as anticancer agents based on their anti-inflammatory, anti-proliferative, and pro-apoptotic effects, despite varying biological mechanisms of action. Long-term use of low-dose aspirin has been associated with a reduced risk of HCC in large-scale observational studies [6]. Statins have also been suggested to reduce the risk of HCC in a number of observational studies and meta-analyses [7-9]. However, they are often under-prescribed in patients with chronic liver disease or cirrhosis due to concerns of bleeding or hepatotoxicity [10,11]. This review outlines current evidence of the chemopreventive effects of aspirin and statins, and provides a current perspective and prospect.
STATINS AND HCC
Mechanism of action
Statins competitively inhibit 3-hydroxy-3-methylglutaryl coenzyme A (HMG-COA) reductase, blocking the conversion of HMG-COA to mevalonate, the rate-limiting step of cholesterol synthesis. As a consequence, statins prevent the synthesis of other important isoprenoid intermediates such as farnesyl pyrophosphate and geranyl pyrophosphate, and reduce intracellular cholesterol synthesis. These isoprenoid intermediates act as important lipid anchors for a variety of proteins including small guanosine triphosphate (GTP)-binding proteins. The pleiotropic effect of statins extends beyond cholesterol reduction and is mediated via inhibition of small GTPase isoprenylation and its downstream signaling pathway [12]. Previous studies showed that statin treatment significantly decreased hepatic inflammation and fibrosis via inhibition of both RhoA/rho kinase and Ras/ERK pathways [13]. The direct anti-inflammatory effect of statins was mediated via decreased levels of interleukin-6 and downregulation of metalloproteinase activity in hepatocytes [14]. In an animal model of nonalcoholic steatohepatitis (NASH), fluvastatin suppressed the activation and hepatic fibrogenesis of steatosisinduced hepatic stellate cells (HSCs) by decreasing the synthesis of reactive oxygen species (ROS), NF-κB activity and expression of pro-inflammatory genes including collagen, transforming growth factor-β, metalloproteinases-1, and alpha-smooth muscle actin [15]. In cirrhotic rat models, atorvastatin downregulated noncanonical (Shh/RhoA) hedgehog signaling in HSCs and decreased fibrosis and portal pressure [16]. Further, statins induced Krüppel-like factor-2 (KLF-2) in liver sinusoidal endothelial cells, resulting in vasoprotective response by inducing the expression of vasodilator and antithrombotic genes including endothelial nitric oxide synthase (eNOS) and thrombomodulin [17]. KLF-2 also inhibits NF-κB transcriptional activity and regulates inflammation and fibrosis [18]. In addition to their anti-inflammatory and anti-fibrotic effects, statins exhibit a direct chemopreventive effect by blocking oncogenic pathways including Ras-MAPK and PI3K/Akt pathways [19,20]. In addition, statins inhibit the activation of the proteasome pathway, limiting the degradation of cyclin-dependent kinase inhibitors p21 and p27 inducing G0/G1 cell cycle arrest [21,22]. They block Myc phosphorylation [23], which results in the suppression of cancer proliferation. Furthermore, statins exert anti-angiogenic effect via impaired synthesis of pro-angiogenesis factors such as vascular endothelial growth factor [24].
Epidemiologic studies: statins and HCC in general population
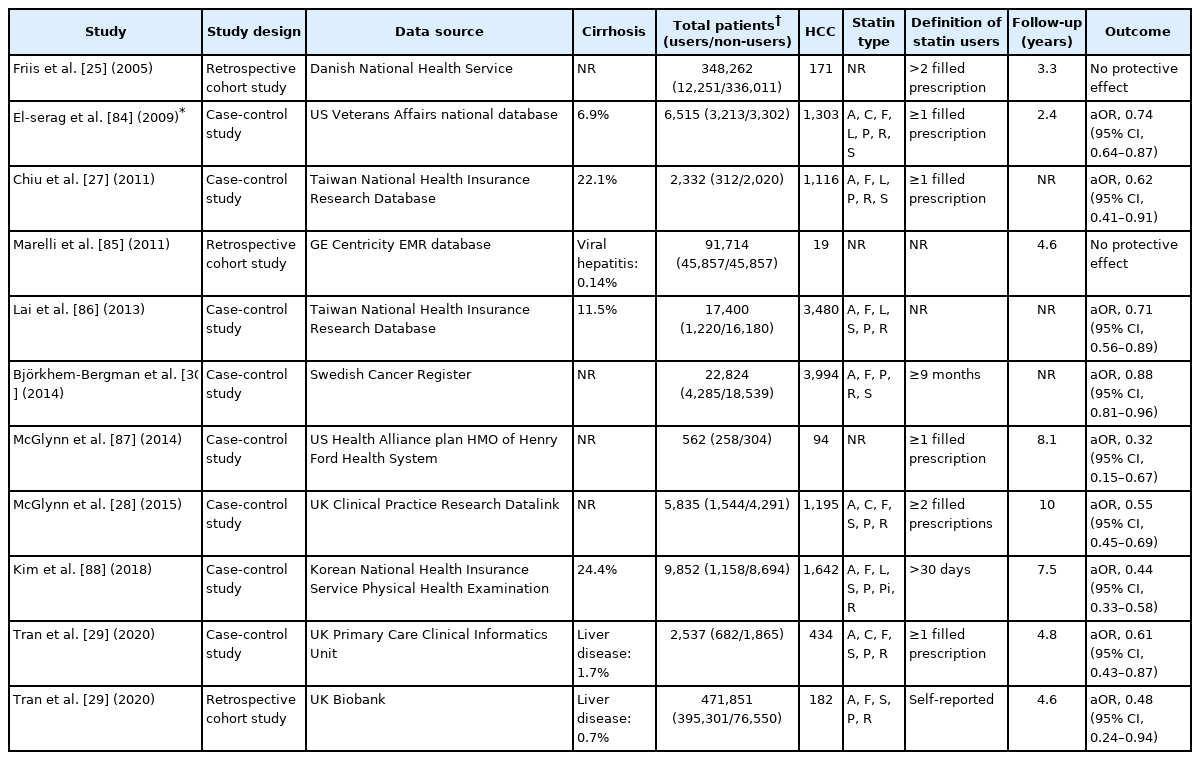
A number of large-scale observational studies using nationwide cohort data have investigated the association between statin use and HCC risk in general population (Table 1). The first cohort study using a Danish National health service database of 348,262 individuals found no significant association between statin use and risk of any cancers including liver cancer (hazard ratio [HR], 1.16; 95% confidence interval [CI], 0.46–2.90) [25]. Post hoc analyses of 134,537 participants from 22 randomized controlled trials (RCTs) in the Cholesterol Treatment Trialists’ collaboration investigating the role of statins in reducing cancer risk failed as well (HR, 1.06; 95% CI, 0.65–1.70) [26]. However, these studies were limited by insufficient follow-up duration of less than 5 years and low incidence of primary liver cancer, which reduced the statistical power of the analysis of statin effects on primary liver cancer. Further, cancer development was a secondary outcome in all RCTs of statin use, which was not systematically investigated, thereby resulting in ascertainment bias.
Clinical studies investigating the effects of statin use on development of hepatocellular carcinoma in general population
A matched case-control study using a large cohort of Taiwan National Health Insurance Research Database found a significant inverse association between statin use and HCC (odds ratio [OR], 0.62; 95% CI, 0.42–0.91) [27]. Subsequent studies using large national cohorts including the Swedish Cancer Resister, UK’s Clinical Practice Research Datalink and the Korean National Health Insurance consistently reported the chemopreventive effects of statins in HCC among the general population regardless of study location [28-30]. Consistent with previous studies, a recent meta-analysis of 11 studies involving the general population reported a 46% reduction in HCC risk among statin users; however, substantial heterogeneity (I2=96.8%) was observed [31].
Epidemiologic studies: statin use and HCC in populations at risk
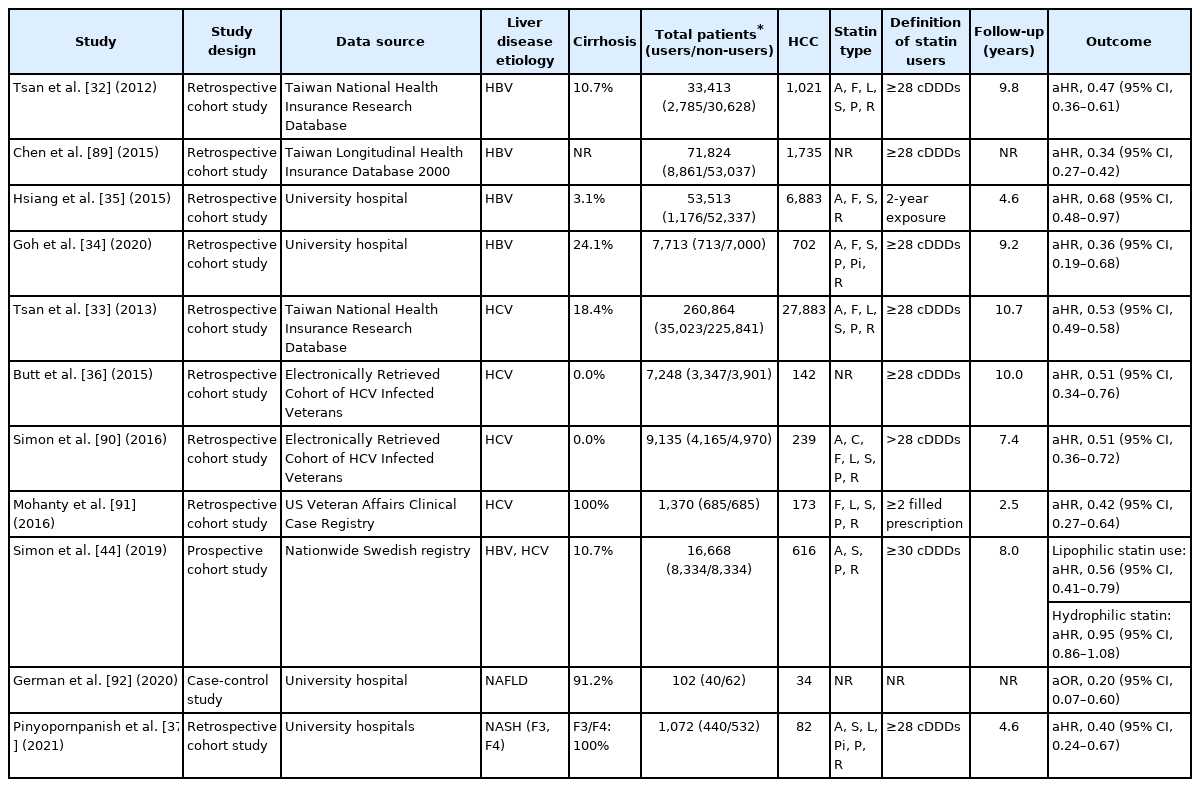
The effects of statins were mainly assessed in patients diagnosed with viral hepatitis including HBV or HCV infections (Table 2). All investigations were performed in retrospective cohorts and no RCTs have been reported. The population-based cohort study using the Taiwan National Health Insurance Reach Database first reported that statin use may reduce the risk of HCC in HBV-infected patients in a dose-dependent manner (adjusted HRs, 0.66, 0.41, and 0.34 for statin use of 28 to 90, 91 to 365, and more than 365 cumulative defined daily doses [cDDDs], respectively) [32]. Subsequently, similar results were reported with 260,864 HCV-infected patients enrolled in the same database (adjusted HRs, 0.66, 0.47, 0.33 for statin use of 28 to 89, 90 to 180, and >180 cDDDs per year, respectively) [33]. Several studies, mostly performed in Asia, reported consistent findings. Furthermore, the protective effect of statin use was consistent or even more potent among patients with HBV treated with NA [34,35]. Butt et al. [36] investigated the impact of statins in patients who received HCV treatment in a longitudinal, national cohort of HCV-infected veterans, the electronically retrieved cohort of HCV-infected veterans. Statin was associated with a significant increase in sustained virologic response (OR, 1.44; 95% CI, 1.29–1.61), a lower risk of progression to cirrhosis (HR, 0.56; 95% CI, 0.50–0.63) and HCC development (HR, 0.51; 95% CI, 0.34–0.76). A meta-analysis of four studies consisting of either HBV- or HCV-infected patients found a significant risk reduction of HCC among statin users with low heterogeneity (pooled HR of patients with HBV, 0.54; 95% CI, 0.45–0.64; I2=37%; pooled HR of patients with HCV, 0.47; 95% CI, 0.42–0.54; I2=0%) [8].
Clinical studies investigating the effects of statin use on population at risk of hepatocellular carcinoma
Few studies investigated the association between statin use and NAFLD-related HCC despite the indication for statin treatment in many of those patients. The most recent large retrospective study conducted at two tertiary academic centers in the United States including 1,072 patients with NASH-related advanced liver fibrosis reported a marked protective effect of statin use against HCC (HR, 0.40; 95% CI, 0.24–0.67) [37]. A dose-dependent response was also observed among statin users with each yearly increment of cDDDs reducing the HCC risk by 23.6% compared with non-users.
A recent meta-analysis of pooled data based on contemporary observational studies involving the general population or at-risk population revealed no significant difference in risk reduction of HCC between general population and high-risk patients (HR, 0.54; 95% CI, 0.42–0.89 vs. HR, 0.52; 95% CI, 0.37–0.73) [31]. In addition, a meta-analysis of six observational studies, which reported the proportion of cirrhotic patients, suggested a consistent HCC reduction regardless of cirrhosis [38].
The effect of statin type
Statins can be categorized into hydrophilic and lipophilic types depending on their solubility. The predominantly lipophilic statins (simvastatin, fluvastatin, pitavastatin, lovastatin, and atorvastatin) enter cells via passive diffusion and are widely distributed in tissues, whereas the uptake of hydrophilic statins (rosuvastatin and pravastatin) entails a liver-specific, carrier-mediated mechanism [39]. Hence, it is hypothesized that lipophilic statins are more pleiotropic due to their non-lipid effects on extrahepatic tissue. Compared with hydrophilic statins, lipophilic statins not only prevent viral replication and stimulate antitumor immunity more effectively [40], but also show potent antitumor effects [41] mediated by G0/G1 cell cycle arrest, inhibition of Ras/Raf/Mek/ERK signaling and apoptosis in preclinical studies [42,43].
Consistent with preclinical data, a recent Swedish study using propensity score-matched cohort of 16,668 adults diagnosed with viral hepatitis in a nationwide population-based cohort reported that the use of lipophilic statins significantly reduced HCC incidence (HR, 0.56; 95% CI, 0.41–0.79). In contrast, no association between hydrophilic statin use and HCC risk was found (HR, 0.95; 95% CI, 0.86–1.08) [44]. Findings from several observational studies and meta-analysis were in agreement with previous studies [45,46]. Conversely, another meta-analysis of individual statin types reported that rosuvastatin, a hydrophilic statin, showed the most pronounced risk reduction in HCC development [7]. The authors assumed that the higher affinity of rosuvastatin for HMG-COA reductase and a greater reduction in cholesterol level when compared to other statins resulted in greater therapeutic effects [7]. Therefore, the beneficial effects related to statin solubility are not supported by robust evidence.
The effect of statin dose and duration
A higher dose of statin was associated with greater risk reduction of HCC development in most studies, while two studies from Hong Kong and Taiwan showed no significant dose-response relationship [27,35]. A two-stage dose-response meta-analysis of six studies investigating statin use and primary liver cancer risk found that an increase in statin dose by every 50 cDDDs per year reduced the risk of primary liver cancer by about 14% [47]. Other dose-response meta-analyses comprising 11 studies found an interesting non-linear dose-response curve suggesting a dose-response relationship between statin dose and a lower risk of primary liver cancer below 100 cDDDs annually or above 200 cDDDs each year. However, no such association existed between 100 and 200 cDDDs per year (HRs, 0.65, 0.60, 0.46 and 0.22 for 55, 200, 320, and 500 cDDDs per year, respectively) [7]. The biphasic effects of statins on angiogenesis described in preclinical studies strengthened their dose-dependent effect [48].
Safety of statin use
Due to the risk of hepatotoxicity, physicians are less likely to prescribe statins for patients with liver disease [10,11]. In fact, drug-induced liver injury related to statins is uncommon (<1.2/100,000 users) and likely idiosyncratic in nature [49]. In contrast, the most common toxicity associated with statins and the leading cause of statin discontinuation is statin-associated muscle symptoms (SAMS), which can manifest as myalgia, myopathy, myositis with elevated creatinine kinase (CK), or rhabdomyolysis [50]. Despite the complex pathogenic mechanisms underlying SAMS, including mitochondrial toxicity, calcium signaling and genetic factors [50,51], the risk of SAMS appears to be linked to systemic exposure to higher doses. As a consequence, individuals with advanced cirrhosis may be at higher risk of SAMS due to increased drug exposure caused by delayed statin clearance, impaired CYP3A4 metabolism in the liver or diminished MRP2 membrane transporter activity [52].
Several observational studies reported no significant differences in drug-induced liver injury or myotoxicity between statin users and nonusers [33,53]. In three of four small RCTs investigating the efficacy of statins on portal hypertension in patients with cirrhosis, no serious adverse events related to statins were reported. However, two patients receiving simvastatin 40 mg/day experienced rhabdomyolysis in one RCT compared with none in the placebo group [54]. The 2014 Assessments updated by the Liver Expert Panel of National Lipid Association Safety Task Force stated that chronic liver disease or compensated cirrhosis is not a contraindication to statin medication. Decompensated cirrhosis or acute liver failure, however, are contraindications for statin use [55].
Statin and HCC: level of evidence
Statins provided consistent chemopreventive benefits against HCC in a variety of study designs with a heterogeneous population. An umbrella systematic review of 43 meta-analyses reported suggestive evidence for four malignancies in patients who used statins: esophageal cancer, hematological cancer, leukemia, and liver cancer [56].
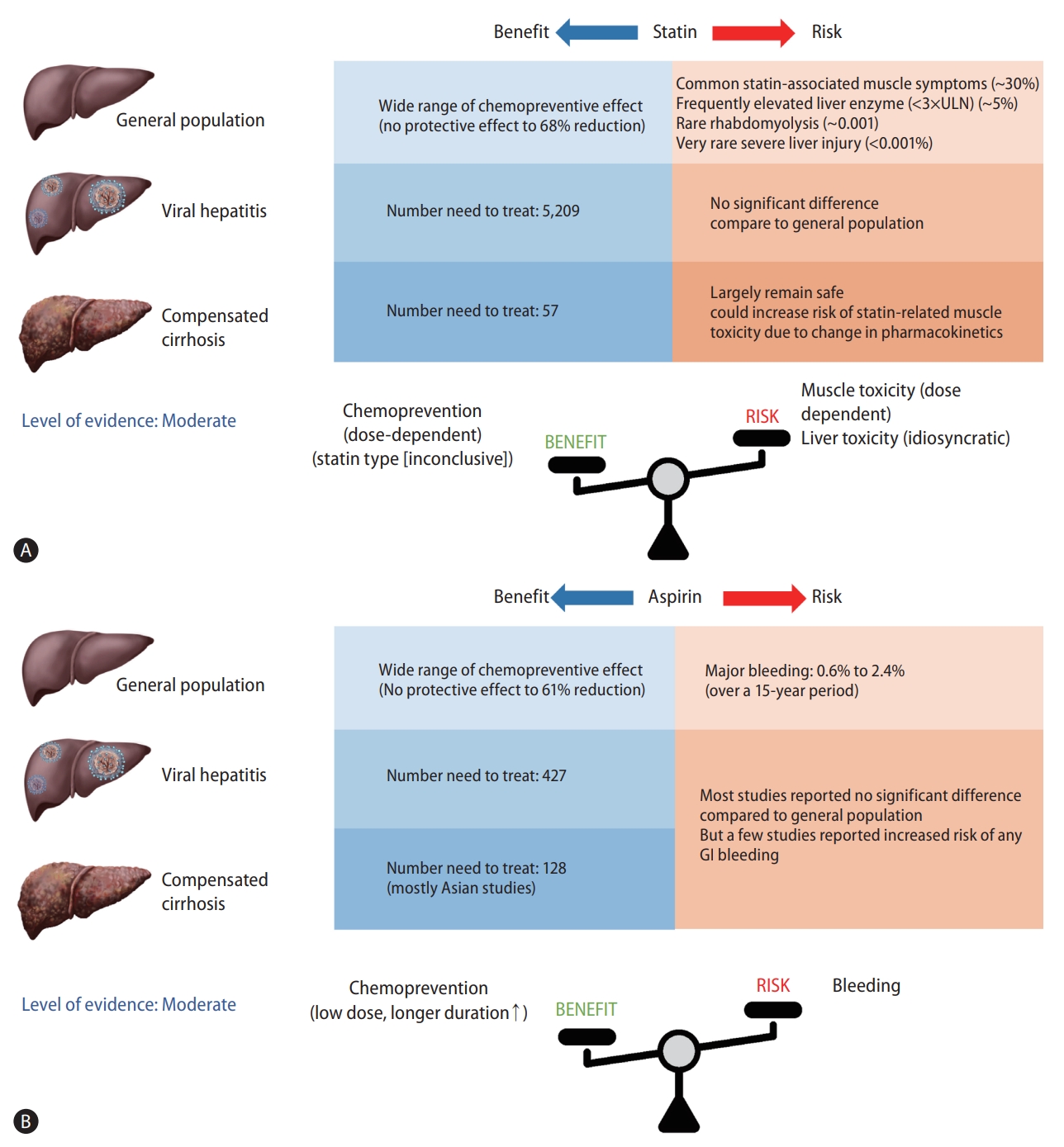
The number needed to treat (NNT) is a widely used metric of clinical benefit that reflects the number of patients who should be treated in order to avoid another adverse event, despite the possibility of misinterpretation depending on baseline risk for HCC. NNT was calculated in 5,209 East Asian males (incidence rate of HCC, 0.04 per 100 person-years) who needed statin therapy to prevent one HCC event per year and in 57 Asian men with HBV-associated cirrhosis who reported an estimated HCC incidence rate of 3.7 per 100 person-years according to a meta-analysis [9]. It is assumed that those with the highest HCC risk are thought to benefit the most from statin chemoprevention.
However, observational studies have limitations due to confounding by indication, other residual confounders, selection bias and immortal time bias leading to overestimation of preventive effect of statins [57]. Furthermore, safety issues should be addressed as the risk of statin-associated toxicity increases in high-risk individuals. Therefore, further prospective RCT data are needed including two ongoing clinical trials (NCT02968810 and NCT03024684) to establish the risk-benefit profile of statins for HCC prevention before they can be recommended for prevention.
ASPIRIN AND HCC PREVENTION
Mechanism of action
Aspirin is an antiplatelet drug that inhibits both isoforms of cyclooxygenase (COX; COX-1 and COX-2), resulting in reduced levels of biologically active prostaglandins (PGE2, PGF2α, PGI2) and thromboxane A2 (TXA2) [58]. Low-dose aspirin (75–100 mg) inhibits COX1 irreversibly, whereas high-dose aspirin, similar to other non-steroidal anti-inflammatory drugs (NSAIDs), has analgesic and anti-inflammatory effects by nonspecifically inhibiting COX-2 [58]. Platelets have been shown to stimulate inflammatory and immune cells in immune-mediated inflammation induced by chronic viral hepatitis, facilitating tissue regeneration and carcinogenesis. In the animal model of chronic HBV infection, small and transient platelet aggregation is induced by microcirculation within the hepatic sinusoids, and these aggregates act as docking sites for circulating virus-specific CD8+ T lymphocytes, eventually triggering liver disease [59]. Aspirin suppressed T-cell-mediated inflammation and HCC progression in the mouse model of chronic immune-mediated HCC, but failed to demonstrate a protective effect in a non-immunologically mediated, toxin-induced HCC model [60]. Furthermore, the proinflammatory COX-2 enzyme is overexpressed in cancer-related inflammation including HCC. Activation of COX increases prostaglandin synthesis, which may accelerate cellular proliferation, invasion, and angiogenesis [61]. Aspirin may act as an anti-tumorigenic agent by decreasing platelet aggregation via TXA2 suppression and COX-2 inhibition, which reduces inflammation and induces cellular apoptosis [62].
Epidemiologic studies: aspirin and HCC in general population
Since aspirin protects cardiovascular and cerebrovascular systems via anti-inflammatory and antithrombotic mechanisms, several randomized clinical trials were conducted in 1990s to investigate the effect of aspirin in the prevention of cardiovascular disease. Although such clinical trials were not designed to investigate the relationship between aspirin use and cancer risk, they were subsequently analyzed to determine the association between aspirin use and cancer risk. Despite conflicting results, a meta-analysis of participants from six RCTs of daily low-dose aspirin for primary prevention in the Antithrombotic Trialists’ Collaboration (35,535 participants) reported a 19% reduction in cancer incidence among aspirin users compared with non-users after 3 years of use [63]. However, due to limited sample size, the effect of aspirin on specific cancer types was not explored. Further studies into the chemopreventive effect of aspirin on HCC development were conducted in the general population (Table 3).
Clinical studies investigating the effects of aspirin use on hepatocellular carcinoma in general population
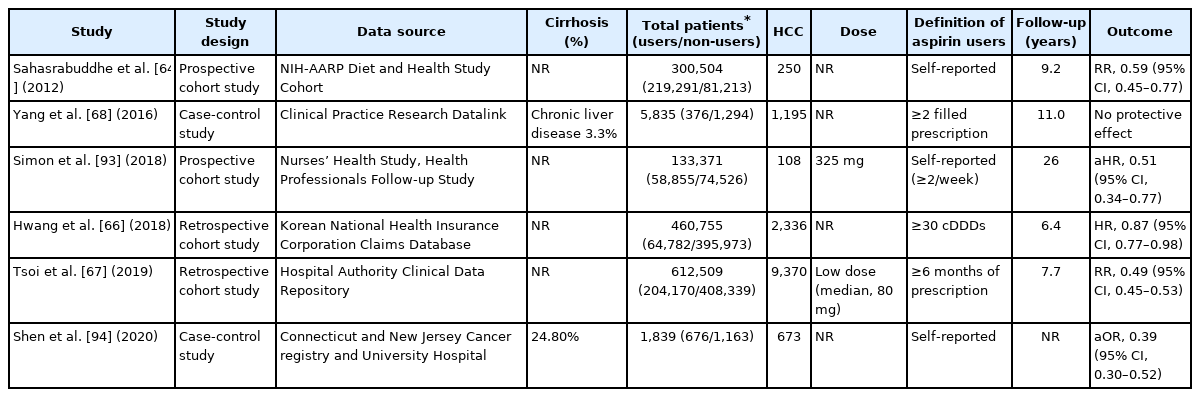
The National Institutes of Health-American Association of Retired Persons (NIH-AARP) Diet and Health study was the first to reveal that that aspirin users had a 41% lower risk of HCC than non-users [64]. As part of the Liver Cancer Pooling Project, a large cohort study of 1,084,133 individuals from ten US-based prospective cohort studies demonstrated that taking aspirin reduced the risk of developing HCC (HR, 0.68; 95% CI, 0.57–0.81) [65]. Furthermore, large national cohort studies from Korea and Hong Kong, both high-risk geographic regions for HCC, found that long-term aspirin use reduced the risk of HCC by 13% and 51%, respectively [66,67].
In contrast, a nested case-control study using data from UK’s Clinical Practice Research Datalink reported a lack of association between the use of NSAIDs including aspirin and liver cancer (OR, 1.11; 95% CI, 0.86–1.44) [68]. In contrast to previous studies that use self-reported data, this study relied on clinical prescription data to determine aspirin use. As aspirin is a widely available over-the-counter medication, particularly in the United States and regions of Europe, studies that rely on prescription claims or medical records may result in exposure misclassification. In fact, when compared with previous cohort studies utilizing self-reported aspirin, a substantially lower proportion of participants used aspirin (28% vs. 73%) [68].
Epidemiologic studies: aspirin and HCC in at-risk patients
Recent investigations into the relationship between aspirin and HCC risk have mainly focused on patients with HCC risk factors such as viral hepatitis or cirrhosis (Table 4), which featured homogenous populations with a higher incidence of HCC. All investigations, similar to those exploring statins, were retrospective, and no RCTs were conducted.
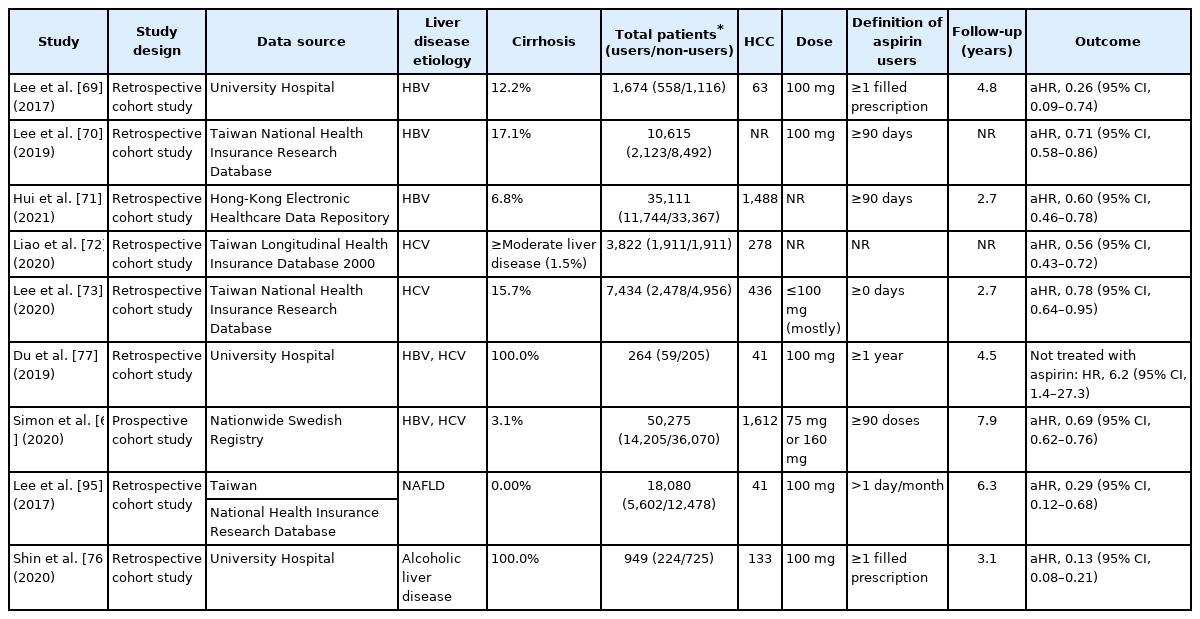
Clinical studies investigating the effects of aspirin use on population at risk of hepatocellular carcinoma
A Korean hospital-based cohort study of 1,624 HBV patients receiving NA treatment reported that antiplatelet therapy was associated with a 56% risk reduction of HCC compared with non-use [69]. Notably, aspirin use was an independent protective factor against HCC (HR, 0.26; 95% CI, 0.09–0.74), whereas neither clopidogrel nor dual antiplatelet therapy showed a significant association (HR, 0.63; 95% CI, 0.15–2.65 and HR, 0.67; 95% CI, 0.28–1.60; respectively) [69]. Similar results were reported by a Taiwan national cohort study; however, the risk reduction was weaker than in the prior study (29% vs. 74%) [70]. The most recent cohort study of 35,111 Hong Kong HBV patients receiving NA treatment reported consistent findings as well as dose-response relationship (HR, 0.65, 0.63, and 0.41 for 0.25–2, 2–5, and ≥5 years, respectively) [71].
According to a cohort study based on Taiwan’s National Health Insurance database, aspirin lowered HCC risk in HCV patients by about 50% [72]. A subsequent Taiwanese cohort study with a higher proportion of HCV-related cirrhotic patients reported consistent findings (HR, 0.78; 95% CI, 0.64–0.95) but no statistical significance was observed in cirrhotic subgroup (HR, 0.75; 95% CI, 0.55–1.03) [73]. Finally, a Swedish nationwide registry-based study in chronic viral hepatitis concluded that low-dose aspirin reduced the risk of HCC and liver-related mortality (HR, 0.69; 95% CI, 0.62–0.76 and HR, 0.73; 95% CI, 0.67–0.81; respectively) [6]. The pooled adjusted HR from seven matched cohort and case-control studies (n=1,799) involving adults with chronic liver disease showed that aspirin use significantly reduced the risk of HCC development (HR, 0.51; 95% CI, 0.36–0.72) [74].
Subgroup analyses of a meta-analysis involving 2.5 million subjects did not significantly alter the risk of liver cancer between general populations (HR, 0.60; 95% CI, 0.56–0.63) and populations with liver disease (HR, 0.66; 95% CI, 0.55–0.80) [75]. Meanwhile, the impact on cirrhotic patients was mixed. Two analyses including a cirrhosis-only population revealed inverse association between low-dose aspirin and HCC risk [76,77] and subgroup analysis of the cirrhotic population (either compensated or decompensated) by Simon et al. [6] showed consistent findings. In contrast, a subgroup analysis using Korean and Taiwanese National Health Insurance database found no association between aspirin and HCC in cirrhotic patients [66,69,70]. Lastly, a recent meta-analysis of individuals with viral hepatitis demonstrated that aspirin use was associated with a lower risk of HCC but the risk reduction rate was lower than in non-cirrhotic patients (HR, 0.85; 95% CI, 0.76–0.95 and HR, 0.64; 95% CI, 0.50–0.83) [78].
Dose and duration
Several studies reported that treatment with low-dose aspirin for a minimum of 3–12 months reduced the HCC risk [71,72]. In contrast, the largest Swedish cohort study of viral hepatitis reported the most favorable outcome for low-dose aspirin after at least 5 years of continued usage, which was associated with a significant reduction in HCC incidence (HR, 0.58; 95% CI, 0.42–0.70) and mortality (HR, 0.63; 95% CI, 0.53–0.75) [6]. Based on dose-response analyses of four studies, each additional aspirin DDD contributed to a significant 0.02% reduction in HCC risk (adjusted RR, 0.98; 95% CI, 0.97–0.98), corresponding to an 8.4% risk reduction per year of daily aspirin use [79]. Another meta-analysis of daily dose response of aspirin based on eight cohort studies found that higher doses exceeding 100 mg/day had no further chemopreventive benefit in incident HCC based on a non-linear model [75].
Safety issue
The benefits of aspirin in primary prevention are offset by higher bleeding risks [80]. Cirrhotic patients frequently manifest coagulation abnormalities and thrombocytopenia, which are associated with an increased risk of bleeding complications. Moreover, the exposure to NSAIDs including aspirin may precipitate hepatorenal syndrome by inducing renal vasoconstriction and lowering glomerular filtration rate [81]. As a result of confounding by indication, data involving potential aspirin-related adverse events in patients with chronic liver disease were limited.
Four retrospective studies involving patients with chronic liver disease found a null association between daily aspirin use and increased risk of gastrointestinal (GI) bleeding [6,69,73,76]. However, a recent meta-analysis of four studies found that aspirin users had a 32% higher risk of GI bleeding (HR, 1.32; 95% CI, 1.08–1.94) than non-users, and patients undergoing antiplatelet therapy (clopidogrel or dual therapy) had more than two-fold higher risk of GI bleeding (HR, 2.62; 95% CI, 1.20–5.85) [74]. Interestingly, a recent study conducted in HBV-infected patients found a duration-dependent risk of GI bleeding after aspirin use. Patients taking aspirin for ≤2 years had a substantially higher risk of GI bleeding (HR, 1.73; 95% CI, 1.07–2.79) than those who did not take aspirin, but this risk decreased after 5 years of usage (HR, 0.79; 95% CI, 0.19–3.21) [71]. Prophylactic usage of a proton pump inhibitor reduces the risk of GI bleeding; however, the risk of spontaneous bacterial peritonitis, hepatic encephalopathy, and Clostridium difficile infection is a concern [82].
Level of evidence
According to the most recent meta-analysis, the pooled HRs from seven matched cohort and case-control studies (n=51,799) investigating the association between aspirin use and HCC risk were 0.51 (95% CI, 0.36–0.72) with moderate evidence based on GRADE certainty. Clinical heterogeneity due to differences in participant characteristics, aspirin dose, duration, concurrent medication usage, and follow-up duration reduced the level of certainty [74].
Another meta-analysis of 19 observational studies involving a total of 2,389,019 individuals estimated that 427 adults with non-cirrhotic chronic HBV infection require aspirin treatment for 1 year to prevent one case of HCC, assuming an HCC incidence rate of 0.6/100 person-years. In the case of cirrhotic patients at a high risk for HCC, with an estimated annual incidence rate of 2%, the NNT to prevent one case of HCC is 128 [79].
However, the NNT in cirrhotic patients requires careful interpretation since the majority of studies investigating the impact of aspirin on HCC prevention in cirrhotic patients were conducted in Asia, with substantial differences in benefit ranging from 22% to 87%. Furthermore, the increased bleeding risk among aspirin users is a clinically important issue in cirrhotic patients, who are at a higher risk of bleeding from esophageal varix, portal hypertensive gastropathy, and even life-threatening bleeding events such as intracranial hemorrhage. As a result, additional prospective RCTs are needed to overcome methodological limitations and to determine the target population for aspirin therapy, demonstrating that the benefits of chemoprevention outweigh the bleeding risks, before aspirin can be recommended as a chemopreventive agent in patients with chronic liver disease.
CONCLUSION
In this review, we have summarized the accumulating data on statins and aspirin for HCC chemoprevention, with a focus on the beneficial effects based on HCC risk, type, dose, and safety (Fig. 1). Statin and aspirin are expected to have a chemopreventive effect, and the potential benefits are supported by preclinical and epidemiological evidence. However, several issues remain to be addressed. First, the vast majority of studies are essentially retrospective in nature and associated with methodological challenges including confounding by indication, residual confounding, selection bias, measurement bias and exposure misclassification. Furthermore, the target population, dose and duration of aspirin and statins whose benefits overweigh harms such as aspirin-induced GI bleeding or statin-associated myopathy were not conclusive. Accordingly, the level of evidence was moderate according to the Oxford Centre for Evidence-Based Medicine 2011 Levels of Evidence [83]. Therefore, additional evidence from prospective RCTs is needed before either statin or aspirin therapy can be recommended for primary prevention of HCC. In contrast, statin or aspirin therapy is not a contraindication in patients with chronic liver disease or compensated cirrhosis and is certainly underutilized in real-life clinical practice. It may be early to use statin or aspirin for chemoprevention purpose. However, statin or aspirin therapy should be actively considered for patients with chronic liver disease or cirrhosis who are indicated for statin or aspirin therapy for other conditions such as prevention of cardiovascular disease.
Notes
Authors’ contributions
MJG: Conceptualization and drafting of the manuscript. DHS: Study supervision and critical revision of the manuscript.
Conflicts of Interest
The authors have no conflicts to disclose.
Abbreviations
cDDDs
cumulative defined daily doses
CI
confidence interval
CK
creatinine kinase
COX
cyclooxygenase
eNOS
endothelial nitric oxide synthase
GI
gastrointestinal
GTP
guanosine triphosphate
HBV
hepatitis B virus
HCC
hepatocellular carcinoma
HCV
hepatitis C virus
HMG-COA
3-hydroxy-3-methylglutaryl coenzyme A
HR
hazard ratio
HSCs
hepatic stellate cells
KLF-2
Krüppel-like factor-2
NAFLD
nonalcoholic fatty liver disease
NAs
nucleos(t)ide analogs
NASH
nonalcoholic steatohepatitis
NIH-AARP
The National Institutes of Health-American Association of Retired Persons
NNT
number needed to treat
NSAIDs
non-steroidal anti-inflammatory drugs
OR
odds ratio
RCTs
randomized controlled trials
ROS
reactive oxygen species
SAMS
statin-associated muscle symptoms
SVR
sustained virologic response
TXA2
thromboxane A2