Incidence and risk factors of dysphagia after variceal band ligation
Article information
Abstract
Background/Aims
There is a lack of data on long-term morbidity, particularly dysphagia, following endoscopic variceal band ligation (EVL). The aim of this study are to assess the incidence of dysphagia and variables associated with this complication after EVL.
Methods
We identified individuals who completed at least one session of EVL as their sole treatment for varices from August 2012 to December 2017. Included patients achieved “complete eradication” of varices not requiring further therapy. Patients ≥90 days from their last EVL session completed a modified version of the Mayo Clinic Dysphagia Questionnaire. Individuals with dysphagia were invited to undergo a barium esophagram. Patients with pre-EVL dysphagia were excluded.
Results
Of the patients, 68 possessed inclusion criteria, nine (13.2%) died and 20 (29.4%) were lost to follow up. For the remaining 39 (57.4%) patients, 23 were males, mean age of 61.7±8.6 years. The most common etiology of liver disease was hepatitis C virus (n=18; 46.2%). The median number of banding sessions was 2.0 (interquartile range [IQR], 1.0–4.0) with a median of 9.0 bands placed (IQR, 3.0–14.0). Twelve patients (30.8%) developed new-onset dysphagia post-EVL. In univariate analysis, pre-EVL MELD score and non-emergent initial banding were associated with long-term dysphagia. In a regression model adjusted for age, sex, number of bands, and use of acid suppression after EVL, no factor was independently associated with dysphagia (all p>0.05). No strictures were identified on subsequent esophageal evaluation.
Conclusions
Approximately 30% of patients developed new-onset, chronic dysphagia post-EVL. Incident dysphagia was associated with a non-emergent initial banding session. The mechanism for dysphagia remains unknown.
INTRODUCTION
Bleeding esophageal varices are the most severe complication of portal hypertension and represent the leading cause of death in patients with cirrhosis. Esophageal varices are present in approximately 50% of patients with cirrhosis [1]. In the past, endoscopic variceal sclerotherapy (EVS) was the intervention of choice in bleeding esophageal varices, but currently endoscopic variceal band ligation (EVL) is thought to be a safer procedure with fewer complications [2,3]. In the immediate setting, EVS can result in bacteremia, which can lead to metastatic infections including pneumonia and bacterial peritonitis [4]. Long-term data has demonstrated that after EVS esophageal stricture formation is relatively common [5]. Additionally, EVS has been associated with esophageal motor disorders including a decrease in esophageal peristaltic wave amplitude and an increase in simultaneous contractions [6,7].
Complications of EVL occur in approximately 14% of cases but are usually minor [8,9]. For example, bacteremia is not seen with EVL. The most common complications are transient dysphagia and chest discomfort [8,9]. These are related to shallow ulcers which develop post-EVL but heal within 4 weeks of treatment [10]. Surprisingly, there is limited long-term data evaluating esophageal symptoms as well as structural and functional abnormalities after EVL. Therefore, the objectives of the study were to: 1) assess the incidence of long-term (>90 days) dysphagia in patients after EVL; 2) identify clinical factors associated with dysphagia; and 3) assess the incidence of esophageal structural disorders after EVL.
MATERIALS AND METHODS
Study population
We identified all patients who underwent upper endoscopy for emergent or elective evaluation and treatment of esophageal varices between August 2012 and December 2017 at our institution. Subjects were identified via the electronic medical record using Provation (ProVation Medical, Minneapolis, MN, USA). Our objective was to identify patients who underwent one or more EVL sessions and at the conclusion were felt to no longer need EVL. Therefore, to be eligible for study inclusion, patients needed to meet all of the following criteria: 1) documented EVL for the treatment of esophageal varices due to portal hypertension; 2) documentation that no further EVL sessions were needed and that only small residual varices were present; and 3) patients must have completed EVL ≥90 days prior to enrollment. We chose a minimum of 90 days to allow for healing of acute ulceration and to allow for the development of fibrotic changes to the mucosa and/or deeper layers of the esophagus post-EVL. We defined dysphagia present >90 days post-EVL as long-term dysphagia. Patients were excluded from the study if they met any of the following criteria: 1) a history of dysphagia prior to EVL; 2) a history of any disorder associated with esophageal dysphagia prior to banding such as Scleroderma and peptic esophagitis/stricture; 3) use of sclerosant during the ablation of varices; and 4) prior gastric or esophageal surgery. Our policy is to perform EVL every 2–4 weeks until varices are obliterated however following this protocol was not a determinant of study inclusion.
Study design
The design was a single-center study. Patients were identified retrospectively but interviewed prospectively. Patients meeting the study’s inclusion/exclusion criteria were interviewed in the office. The study protocol was described and informed consent was obtained which was approved by Institutional Review Board (IRB) of Temple University School of Medicine (IRB No. 23996). Eligible patients were administered a modified version of the Mayo Dysphagia Questionnaire (MDQ) [11]. The full MDQ contains 28 items however we retained only those questions relevant to our study. The retained eight questions focus solely on dysphagia (Fig. 1). If question 1, “Do you have trouble swallowing solid food?” was answered with “yes”, the patient was considered to have dysphagia. The remaining questions quantify the frequency of dysphagia and types of foods the patient finds troublesome to swallow. From the questionnaire, patients identified as having dysphagia were invited to undergo a barium esophagram which included ingestion of a 13 mm barium-impregnated pill.
Demographic data such as age, sex, and etiology of liver disease were recorded. We recorded the model for end-stage liver disease (MELD) score and Child-Turcotte-Pugh class for patients both pre- and post-EVL. Endoscopic variables collected included the presence of a hiatal hernia, whether the original bands were placed electively or emergently, number of banding sessions, total number of bands placed, and whether the patient was placed on a proton pump inhibitor after the procedure. We reviewed each study to determine whether an esophageal stricture was noted during or after banding.
Statistical analysis
We performed a univariate analysis comparing variables between subjects who did and did not develop dysphagia after banding. Comparison of continuous data was performed using Student’s t-test after confirming a normal distribution of data points. For categorical variables, a chi-square or Fisher Exact test was used. For non-parametric data, appropriate formulas such as Mann-Whitney U or Kruskall-Wallis were used. All analyses were 2-tailed with a significance level (α) set at 0.05.
All significant (P≤0.05) predictors of dysphagia from univariate analysis were entered into a logistic regression model. Using dysphagia as the outcome (dependent variable), a stepwise forward entry of predictor (independent) variables was performed to determine if any variables were independently associated with dysphagia. Data output was expressed as the adjusted odds ratio with corresponding 95% confidence interval (CI). SPSS Statistics ver. 24 (IBM, Armonk, NY, USA) was used for statistical analysis.
RESULTS
During the period of August 2012 and December 2017, 68 patients met study inclusion/exclusion criteria. However, nine (13.2%) expired and 20 (29.4%) were lost to follow-up (Fig. 2). For the evaluable 39 (57.4%) patients, there were 23 (59%) males and 16 (41%) females with a mean age of 61.7±8.6 years (Table 1). The major etiologies of liver disease included hepatitis C virus (HCV) (n=18; 46.2%), non-alcoholic steatohepatitis (n=7; 17.9%), and alcoholic liver disease (n=7; 17.9%). For those with HCV a similar proportion had viral eradication prior to EVL. The median number of banding sessions, including the index session if performed for bleeding, was 2.0 (interquartile range [IQR], 1.0–4.0), with a range of 1–5 sessions. The median total number of bands placed per patient was 9.0 (IQR, 3.0–14.0), with a range of 1 to 30 bands.
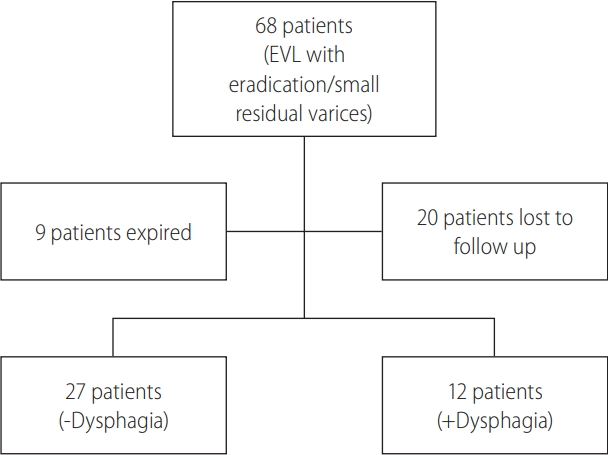
Flow diagram of patient inclusion in the study. Patients with dysphagia answered “Yes” to the question “Do you have trouble swallowing solid food?”. EVL, endoscopic variceal band ligation.
Twelve patients (30.8%) developed new onset dysphagia post-EVL. The table compares the characteristics of patients who did and did not develop dysphagia. For those with dysphagia, with respect to difficulty swallowing food in the past 30 days, 50% had difficulty once per week, 25% less than once per week, and 16.6% several times per week. To prevent food “getting stuck”, 58.3% avoided meat, 25% avoided apples, and 25% avoided ground meat, with smaller percentages for other foodstuffs. Asked another way, 66% had trouble swallowing meat, 33% had trouble swallowing ground meat, and 25% had trouble swallowing apples. When asked whether post-EVL dysphagia sufferers modified their diet to make it easier to swallow, 50% answered “yes”, 50% “no”. With respect to liquid dysphagia, 91.7% responded “no” and 8.3% “yes”. For difficulty swallowing pills, 66% responded “no” and 33% “yes”.
On univariate analysis, the variables associated with dysphagia post-completion of EVL were the proportion of cases where the initial procedure was performed non-emergently vs. emergently (40.7 vs. 8.3%, P =0.043) and the pre-EVL MELD score for those with (10.8±3.0) and without (15.0±6.8) dysphagia (P =0.01). We performed a stepwise forward binary logistic regression, controlling for age, gender, total number of bands placed, and whether proton pump inhibitor therapy was used after EVL. After adjustment, both pre-EVL MELD score (adjusted odds ratio [OR], 0.84; 95% CI, 0.69–1.02; P =0.07) and whether the first banding session was emergent (adjusted OR, 8.32; 95% CI, 0.74–93.6; P =0.08) were no longer significantly associated with post-EVL dysphagia (Table 2).
Evaluation for esophageal stricture
None of the twelve cases of new onset dysphagia post-EVL were identified to have a stricture on their final upper endoscopy as part of EVL treatment. Only five of 12 agreed to undergo an esophagram post-EVL; three were normal, one showed a diffuse abnormality of peristalsis, and one demonstrated a focal mural irregularity without luminal narrowing in the distal esophagus. One patient underwent upper endoscopy for another indication 16 months after completing EVL and no stricture was identified.
DISCUSSION
The current study investigated the incidence and potential variables associated with dysphagia in patients ≥90 days after complete eradication of all varices with band ligation. At this point in time, we know that there is long-term treatment-related morbidity associated with EVS (approximately 40%) and short-term morbidity with EVL including transient dysphagia [12,13]. Our study is a unique investigation because the prevalence of long-term complications from EVL has not been carefully studied.
We explored several relevant potential risk factors for dysphagia post-EVL including age, sex, number of banding sessions, number of bands placed, and whether the index EVL was performed emergently. The median number of EVL sessions in our study to achieve eradication was 2.0, similar to findings in other studies [14]. We found on univariate analysis that a lower MELD score and whether the index EVL was performed non-emergently for bleeding were predictors of experiencing dysphagia as a long-term morbidity. However, after controlling for relevant potential confounders using logistic regression modeling, neither of these variables was shown to be independently associated with post-EVL dysphagia.
In 1989, Van Stiegmann and Goff [9] and Van Stiegmann et al. [15] introduced the application of EVL. In their study they identified that all of their patients achieving complete variceal eradication reported mild dysphagia for 12–24 hours following EVL but none required active treatment or developed strictures. The results of the study highlighted that EVL is a safe alternative to EVS but did not look at long-term complications. Similarly, Viazis et al. [4], compared the short-term effects of EVL and EVS after variceal eradication on esophageal motility. This randomized study of 60 patients found that none treated with EVL (n=30) complained of reflux symptoms or dysphagia after completion of endoscopic treatment but follow-up was only for 6 weeks.
The technique used for EVL obliterates varices by causing mechanical strangulation with rubber bands, an adaptation of the concept applied to band ligation of internal hemorrhoids. The main reaction is usually limited over the superficial esophageal mucosa where ischemic necrosis occurs, followed by granulation tissue formation and sloughing of the bands [13]. This may lead to shallow mucosal ulcerations and complete re-epithelialization of the mucosa with maturing scar tissue that can heal over time [16]. We chose ≥90 days after the patient’s final EVL session as this should allow for sufficient time for edema and superficial mucosal ulceration to resolve. Additionally, we assumed early stricture formation would be present by this time if it were going to develop.
In our study we identified that nearly one third of patients developed new-onset dysphagia ≥90 days post-EVL when a modified version of the Mayo Dysphagia Questionnaire was administered. 11 This is novel information as again, it is common to have transient dysphagia immediately post-banding, however the assumption has been that the dysphagia will resolve over time. While dysphagia prevalence was high, there was no evidence that EVL caused significant structural abnormalities of the esophagus for those that completed a barium esophagram or upper endoscopy.
Because stricture formation post-EVL appears to be very rare, the etiology of dysphagia remains elusive. Esophageal motility was not systematically evaluated in this study although one patient was shown to have a diffuse, non-specific peristaltic disorder of the esophageal body post-EVL on barium esophagram. In contrast, data from Viazis et al. [4], demonstrated that EVL has no impact on esophageal motility unlike EVS, which the authors found leads to a significant decrease of the amplitude of peristaltic waves, an increase of simultaneous contractions in the tubular esophagus, and an increase in pathological gastroesophageal reflux disease. Similarly, a case series published by Berner et al. [17], demonstrated that the majority of EVL treatments did not result in a worsening of motility or reflux scores and none were associated with symptoms. This study included six patients with Child’s class B cirrhosis and one patient with pre-sinusoidal portal hypertension for which the effects on esophageal motor function were evaluated both pre- and post-EVL [17]. Whereas band ligation appears to have little impact on esophageal motility data are limited and hampered by lack of standardization rendering conclusions about safety premature [18]. Larger studies that evaluate patients for esophageal motility disorders at least 3–6 months after EVL are needed.
There are some limitations to our study. While we identified 68 people who completed EVL, a number were lost to follow up after treatment ultimately decreasing our sample size. Second, our study only looks at EVL morbidity without an EVS arm. However, a prospective, randomized trial comparing EVL with EVS to evaluate long-term complications such as dysphagia would be difficult to perform given that most institutions no longer perform EVS routinely. Third, this was a single center study with a relatively small number of patients. Involving multiple institutions may allow for a more broad application of our findings. In addition, we incorporated a modified version of the MDQ that has not been previously validated. We chose this method of categorizing symptoms because no other suitable questionnaire for our purposes existed. Other studies have had this similar limitation such as studies published by Viazis et al. [4] and Goff et al. [8], where both studies utilized ad hoc dysphagia questionnaires.
In summary, dysphagia appears to be a common, long-term complaint a physician may encounter post-EVL. We were unable to identify any independent predictors of post-EVL dysphagia in regression analysis which controlled for several relevant potential confounders. Our study is important as only short-term esophageal symptoms after EVL have been previously reported. The mechanism resulting in dysphagia needs to be further clarified in larger, long-term studies.
Notes
Authors’ contribution
Saraswathi Arasu: study concept and design; data entry; analysis and interpretation of data; drafting of manuscript
Hammad Liaquat: data entry; analysis and interpretation of data
Jaspreet Suri: study concept and design; data entry; analysis and interpretation of data; drafting of manuscript
Adam Ehrlich: study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; study supervision
Frank Friedenberg: study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; study supervision
Notes
Conflicts of Interest: The authors have no conflicts to disclose.
Abbreviations
CI
confidence interval
EVL
endoscopic variceal band ligation
EVS
endoscopic variceal sclerotherapy
HCV
hepatitis C virus
IQR
interquartile range
MDQ
Mayo Dysphagia Questionnaire
MELD
model for end-stage liver disease
OR
odds ratio
References
Article information Continued
Notes
Study Highlights
This investigation evaluated the prevalence of dysphagia post-endoscopic variceal band ligation (EVL) and identified variables that may be associated with dysphagia. Our study showed that nearly 1/3 of patients developed new onset dysphagia post-EVL; however, there were no variables independently associated with this outcome in logistic regression analysis. This manuscript will be of interest to not only clinical investigators in the area of the esophagus, but also to physicians caring for the many patients who have decompensated cirrhosis requiring esophageal variceal band ligation.



