Interferon-free treatment for hepatitis C virus infection induces normalization of extrahepatic type I interferon signaling
Article information
Abstract
Background/Aims
Hepatitis C virus (HCV) replicates in the peripheral blood mononuclear cells (PBMCs), leading to the production of type I interferons (IFNs). It is well known that the gene expression profile of PBMC is similar to that of the liver. The present study explored the dynamic gene expression profile of PBMCs collected from HCV-infected patients undergoing direct-acting antiviral (DAA) therapy.
Methods
A prospective cohort comprising 27 patients under DAA therapy was formed. Expression level of IFN-β and its downstream interferon-stimulated genes (ISGs) was measured in PBMCs before and after DAA treatment. Furthermore, immunoblotting was performed to identify the signaling molecules involved in the expression of ISGs.
Results
The pretreatment expression level of interferon-induced protein 44 (IFI44) and C-X-C motif chemokine ligand 10 (CXCL10) correlated with the pretreatment expression level of IFN-β. After DAA treatment, a significant decrease in the expression levels of IFN-β, IFI44, and CXCL10 was observed in the PBMCs. Furthermore, the pretreatment expression level of IFN-β and ISGs correlated with the level of signal transducer and activator of transcription 1 (STAT1) phosphorylation, and DAA treatment abrogated STAT1 phosphorylation.
Conclusions
Pretreatment activation of IFN-β response is rapidly normalized after DAA treatment. The present study suggests that the decreased type I IFN response by the clearance of HCV might contribute to DAA-induced alleviation of extrahepatic manifestation of chronic HCV infection.
INTRODUCTION
Hepatitis C virus (HCV) is a positive-stranded RNA virus, and more than 184 million people are infected with HCV worldwide [1]. After HCV infection, most of the patients fail to clear the virus from the blood and develop chronic persistent infection [2]. Furthermore, HCV infection often leads to the development of hepatic complications, such as cirrhosis and hepatocellular carcinoma. Up to 70% of the patients with chronic HCV infection experience extrahepatic manifestations [3].
Many types of interferons (IFNs) have been identified [4,5]. Upon binding of IFNs to their respective receptors, cellular actions are mediated through specific interferon-stimulated genes (ISGs) with antiviral and immunoregulatory effects [5]. Currently, IFNs are classified into three major classes: type I, type II, and type III. Each class of IFNs signals to the host cell by binding to different receptor complexes. Type I IFN comprises 13 IFN-αs, in addition to IFN-β, IFN-ω, IFN-ε, and IFN-κ [5]. Type I IFNs are critical mediators of inflammation and immunosuppression during chronic viral infection [6]. The control of these infections requires type I IFN, although prolonged exposure results in the dysfunction of immune cells [6].
After the establishment of HCV infection, type I and III IFNs are endogenously produced by the HCV-infected cells [7]. Consequently, type I and III IFNs after binding to their respective receptors initiate a signaling cascade through the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway [7,8]. The cellular actions are mediated by the induction of ISGs. Our recent study demonstrated that endogenous type I and III IFNs are produced in response to HCV infection, thus rendering the cells nonresponsive to exogenous IFN-α [9,10]. Furthermore, treating the HCV-infected primary human hepatocytes with telaprevir and sofosbuvir (SOF) reduced the intracellular HCV RNA titer resulting in the attenuated induction of ISGs and restoration of IFN-α responsiveness [10].
Previous studies have reported that HCV can replicate in the peripheral blood mononuclear cells (PBMCs) [11-13]. A recent study reported that G28A variant HCV replicates in the PBMCs independent of microRNA (mir)-122 [11]. It has also been shown that this variant is associated with extrahepatic manifestation of chronic HCV infection [11]. Furthermore, another report showed that the pattern of ISG expression is similar in the liver and PBMCs of HCV-infected patients [14]. These reports suggest that HCV can replicate in the PBMCs, and induce a pattern of ISG expression similar to that in the liver.
Recently, various types of direct-acting antivirals (DAAs) against HCV have been developed. A high rate of sustained virologic response (SVR) is achieved by DAAs without the addition of IFNs [15]. As a result, the standard of care for chronic HCV infection has shifted from IFNα-based therapies to DAAs [16]. The present study explored the dynamic gene expression profile of PBMCs collected from HCV-infected patients treated with DAA therapy. The expression level of IFN-β and ISGs was analyzed before and after DAA treatment, and correlated the expression level with the level of STAT1 phosphorylation and clinical parameters.
PATIENTS AND METHODS
Patients
From March 1 to September 30 2016, 51 patients with HCV genotype 1b infection receiving dual oral therapy with daclatasvir (Daklinza®, Bristol Myers Squibb, New York, NY, USA) and asunaprevir (Sunvepra®, Bristol Myers Squibb, New York, NY, USA) for 24 weeks (wks) at the Division of Hepatology in the Seoul St. Mary’s Hospital were examined. Among them, 21 patients agreed to donate blood samples and were enrolled in the present study. Five patients infected with HCV genotype 2 were also enrolled. They were treated with SOF (Sovaldi®, Gilead, Foster City, CA, USA) and ribavirin. One patient infected with HCV genotype 1a was enrolled and was treated with ledipasvir plus SOF (Harvoni®, Gilead, Foster City, CA, USA).
A prospective cohort with these 27 patients was formed, blood samples were obtained, and PBMCs were isolated at the start and end of the treatment. Detailed history taking and physical examination were performed to confirm extrahepatic manifestation of HCV in the enrolled patients. Liver stiffness was measured by transient elastography using the FibroScan® (Echosens, Paris, France), and the result was expressed in kPa. For patients infected with HCV genotype 1, non-structural 5A protein (NS5A) resistance-associated substitution (RAS) testing was performed [17]. Population sequencing (Sanger method) of the full-length HCV NS5A coding region was performed by Seoul Clinical Laboratories (Yongin, Korea). Further, NS5A RASs were defined as the following substitutions at the following positions: L31I/F/M/V and Y93C/F/H/N/S.
SVR12 was defined as undetectable HCV RNA at 12 wk after the completion of treatment. Virologic failure consisted of viral break-through (detectable on treatment with HCV RNA following an undetectable level), relapse (undetectable HCV RNA at the end of treatment, and then quantifiable 12 wk after the end of treatment), and undefined failure (detectable HCV RNA level during all the visits). The HCV RNA copy number in the sera of the enrolled patients was determined.
The present study was conducted according to the Declaration of Helsinki principles and was approved by the institutional review boards (Seoul St. Mary’s Hospital, KC15OISI0787). The written informed consent was received from the participants prior to their inclusion in the study.
RNA extraction, complementary DNA synthesis, and real-time quantitative polymerase chain reaction analysis
The PBMCs were isolated from patients with chronic HCV infection by Ficoll–Hypaque density gradient method [18]. Total RNA isolation, complementary DNA (cDNA) synthesis, and real-time quantitative polymerase chain reaction (PCR) analysis were performed as previously described [19]. Real-time quantitative PCR with 5’-nuclease probes is a relatively robust and precise method for specific DNA sequence detection and quantification with minimal background signal [20]. TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA, USA) were used to determine the level of mRNA of the target genes. The assay ID of each gene is as follows: IFI44 (Hs00197427_m1), CXCL10 (Hs00171042_m1), IFN-β (Hs01077958_s1), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Hs02786624_g1). Real-time quantitative PCR was performed with the following temperature settings: (1) 95°C for 10 min, and (2) 40 cycles at 95°C for 15 s, and 60°C for 30 s. The results were standardized to the mRNA level of GAPDH, and the data were presented as the mean ± standard deviation (SD). To quantify HCV RNA, previously reported and validated primers and probes were used [19].
Immunoblotting
The detailed immunoblotting protocol has been described previously [21]. Pellet of PBMCs was lysed with radioimmunoprecipitation assay (RIPA) buffer, and 7 μg of the cell lysate was loaded onto sodium dodecyl sulfate-polyacrylamide gels. The blotted membrane was blocked in 5% non-fat dry milk diluted in tris-buffered saline for 1.5 h at room temperature. Primary antibodies were diluted in the blocking buffer, and the membrane was incubated with the diluted primary antibodies for 19 h at 4°C. After washing, the membrane was incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies for 30 min at room temperature. After washing, the ImageQuant LAS 4000 (GE Healthcare, Hatfield, UK) was used to obtain the images. The antibodies used are as follows: STAT1 (1:1,000, rabbit, BD transduction laboratories, San Jose, CA, USA), PY-STAT1 (1:1,000, mouse, BD transduction laboratories), GAPDH (1:1,000, rabbit, Santa Cruz Biotechnology, Santa Cruz, CA, USA), HRP-conjugated rabbit immunoglobulin G (IgG) (1:5000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), and HRP-conjugated mouse IgG (1:5,000, Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Statistical analyses
The discrete variables were compared by the χ2 test, and independent t-test was used for continuous variables. Both Pearson and Spearman correlation analyses were used to evaluate the correlation between continuous variables. Statistical significance was defined as a P value < 0.05. SPSS version 20 software (IBM Corp., Armonk, NY, USA) was used for all the analyses.
RESULTS
Study population
Patient demographics were summarized in Table 1. The median age of the patients was 63 years. Non-structural 5A protein RASs were not detected in any of the enrolled patients. Among the patients, 20 patients were treatment-naïve. Two patients were nonresponders and two patients relapsed after treatment with the pegylated IFN plus ribavirin. Three patients were intolerant to the pegylated IFN plus ribavirin treatment and did not complete the schedule. Four patients were diagnosed with liver cirrhosis and 23 patients were diagnosed with chronic hepatitis. The median value of liver stiffness measured by transient elastography was 6.7 kPa. At least one clinical extrahepatic manifestation was observed in 17 of 27 patients (63%). The most common extrahepatic manifestation was fatigue (33%). Type II diabetes (15%), arthralgia (15%), pruritus (11%), depressive mood (11%), chronic kidney diseases (7%), and sicca syndrome (4%) were also observed in the study group. The SVR12 rate was 90.5% (19/21) in patients with HCV genotype 1b infection, 100% (1/1) in patients with HCV genotype 1a infection, and 100% (5/5) in patients with HCV genotype 2 infection.
Treatment-induced downregulation of endogenous IFN-β signaling in the PBMCs of HCV-infected patients
Initially, the mRNA level of IFN-β, IFI44, and CXCL10 in the PBMCs of the cohort before DAA treatment was measured. Further, IFI44 and CXCL10 were chosen, because they were proved as representative ISGs induced by IFN-β in our previous studies [2,9,10]. The pretreatment expression level of IFI44 and CXCL10 correlated with the pretreatment expression level of IFN-β (Fig. 1A). However, the expression level of these ISGs was not associated with the expression of IFN-λ1 (data not shown).
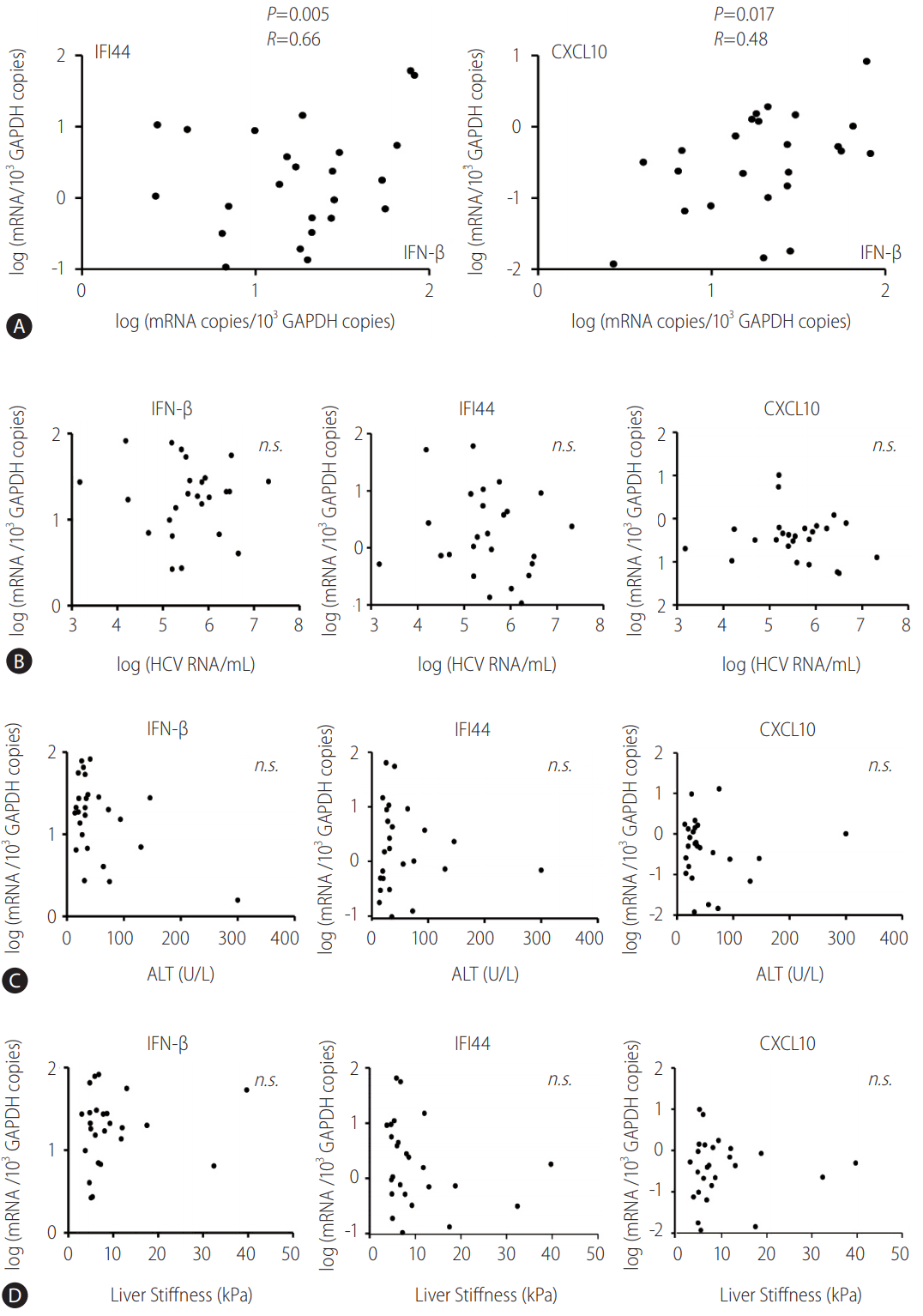
Pretreatment IFN-β expression correlates with the expression of IFI44 and CXCL10 in the peripheral blood mononuclear cells (PBMCs) isolated from HCV-infected patients. (A-D) PBMCs from HCV-infected patients were isolated before direct-acting antiviral treatment. TaqMan real-time quantitative polymerase chain reaction was performed to detect mRNA levels of IFN-β, IFI44, CXCL10, and GAPDH. Pearson's correlation analysis was performed. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IFI44, interferon-induced protein 44; IFN, interferon; CXCL10, C-X-C motif chemokine ligand 10; n.s., not significant; HCV, hepatitis C virus; ALT, alanine aminotransferase.
The present study also verified if the mRNA level of IFN-β, IFI44, and CXCL10 in PBMCs before treatment correlated with the baseline HCV RNA copies, alanine aminotransferase (ALT) level, or liver stiffness. The results revealed that the pretreatment expression level of IFN-β, IFI44, and CXCL10 did not correlate with the baseline HCV RNA copies (Fig. 1B), ALT levels (Fig. 1C), or liver stiffness (Fig. 1D).
Subsequently, the mRNA level of IFN-β, IFI44, and CXCL10 in the PBMCs before DAA treatment was compared with those after DAA treatment. The expression level of IFN-β was significantly downregulated in the PBMCs after DAA treatment (Fig. 2A). Similarly, the expression level of IFI44 and CXCL10 was also downregulated in the PBMCs after DAA treatment (Fig. 2B). Viral kinetics of the enrolled patients was presented in Fig. 2C. There were two patients with viral breakthrough (Fig. 2C), and they did not show a decrease in the expression level of these genes (Fig. 2D).
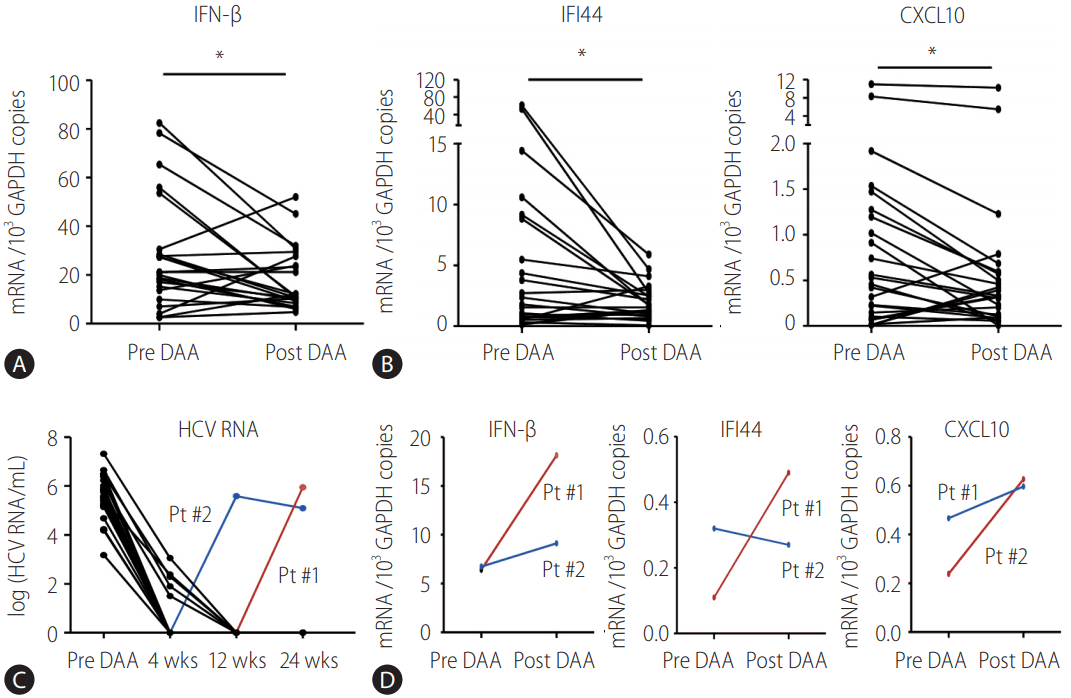
Direct-acting antiviral (DAA)-induced downregulation of endogenous IFN-β response in peripheral blood mononuclear cells (PBMCs) isolated from HCV-infected patients. (A, B) PBMCs from HCV-infected patients were isolated before DAA (pre DAA) and at the end of DAA (post DAA) treatment. TaqMan real-time quantitative polymerase chain reaction (PCR) was performed to detect mRNA levels of IFN-β, IFI44, CXCL10, and GAPDH. (C, D) Serum and PBMC from two HCV-infected patients were isolated before and at the end of DAA treatment. TaqMan real-time quantitative PCR was performed to detect viral RNA level of HCV in the sera and mRNA levels of IFN-β, IFI44, CXCL10, and GAPDH in the PBMCs. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IFN, interferon; IFI44, interferon-induced protein 44; CXCL10, C-X-C motif chemokine ligand 10; HCV, hepatitis C virus; wks, weeks; Pt, patient. *P<0.05.
Level of STAT1 phosphorylation decreased after DAA treatment in the PBMCs of HCV-infected patients
To confirm the decreased activation of JAK-STAT signaling by DAA treatment, immunoblotting of STAT1 and tyrosine-phosphorylated STAT1 (PY-STAT1) proteins was performed using the PBMCs of patients. As expected, the level of STAT1 phosphorylation before DAA treatment correlated with the mRNA levels of CXCL10 (P =0.03, R=0.86) (Fig. 3A, B). However, the level of STAT1 phosphorylation was not significantly associated with IFI44. After DAA treatment, the level of STAT1 phosphorylation decreased (Fig. 3C). These results were presented in Fig. 2, which showed the decreased production of IFN-β after DAA treatment. Overall, the endogenous IFN-β signaling, represented by STAT1 phosphorylation and ISGs induction, was downregulated by DAA treatment in the PBMCs collected from HCV-infected patients.
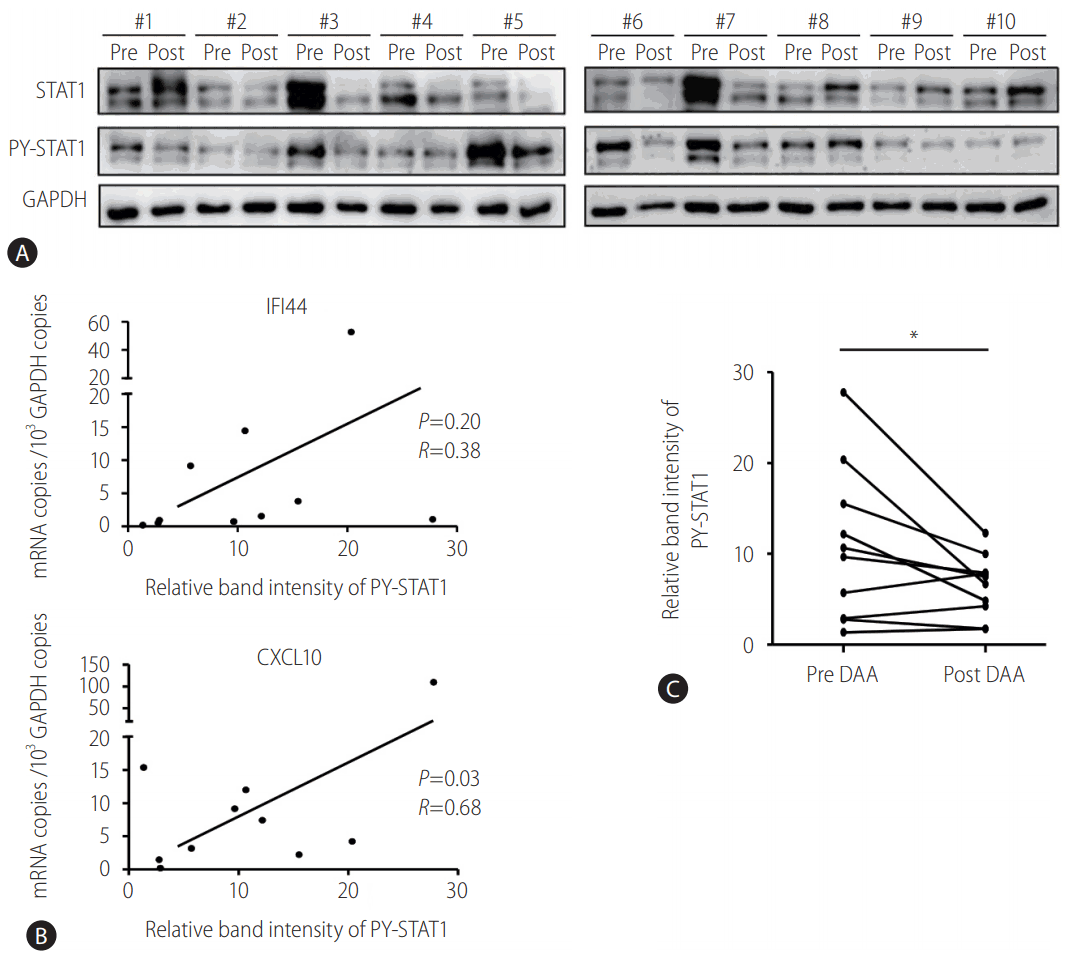
STAT1 phosphorylation decreased in the peripheral blood mononuclear cells (PBMCs) of HCV-infected patients after direct-acting antiviral (DAA) treatment. (A, C) PBMCs from HCV-infected patients were isolated before DAA (pre DAA) and at the end of DAA (post DAA) treatment. Immunoblotting was performed to detect the protein levels of STAT1, PY-STAT1, and GAPDH. (B) PBMCs from HCV-infected patients were isolated before DAA treatment. TaqMan real-time quantitative polymerase chain reaction was performed to detect mRNA levels of IFI44, CXCL10, and GAPDH. Pearson's correlation analysis was performed to identify the associations between expression levels of IFI44/CXCL10 and relative band intensity of PY-STAT1. STAT1, signal transducer and activator of transcription 1; HCV, hepatitis C virus; PY-STAT1, tyrosine-phosphorylated STAT1; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IFI44, interferon-induced protein 44; CXCL10, C-X-C motif chemokine ligand 10. *P<0.05.
DISCUSSION
The present study demonstrated that the pretreatment activation of IFN-β response, reflected by the upregulation of ISGs transcripts and level of STAT1 phosphorylation, is rapidly normalized after DAA treatment.
In HCV-infected cells, double-stranded viral RNA is sensed by retinoic acid-inducible gene I, melanoma differentiation-associated protein 5, and Toll-like receptor 3, thus activating downstream signaling and inducing type I IFNs [2,22]. Intracellular signals from the receptors are transmitted to mitochondrial antiviral signaling protein (MAVS) and Toll/IL-1 receptor domain-containing adaptor inducing IFN-β (TRIF), followed by the induction of IFNs [22,23]. Further, HCV uses mechanisms to interfere with the induction of IFNs. Importantly, HCV NS3/4A protease is known to cleave MAVS and TRIF, consequently blocking the transmission of the intracellular signaling toward type I IFN production [2]. However, HCV cannot completely block the signaling, and it has been demonstrated that HCV infection is associated with the activation of the type I IFN system by the induction of ISGs [24,25]. Continuous upregulation of ISGs in the HCV-infected liver has been reported in chimpanzee models [24,26] and HCV-infected humans [27,28]. Interestingly, HCV RNA and ISG mRNA are detected simultaneously in the hepatocytes of patients with chronic HCV infection [29]. These findings suggest that HCV infection stimulates the induction of endogenous IFNs, which result in the upregulation of ISGs in the infected liver [29]. Because a previous study reported that the expression pattern of ISGs is similar in the liver and PBMCs of HCV-infected patients [14], the present study used the PBMCs of patients to monitor changes in the expression of IFN-β and the induction of downstream ISGs.
Our previous study demonstrated that the expression of CXCL10 is more rapidly induced and better correlates with STAT1 phosphorylation by type I and II IFNs than that of other antiviral ISGs, such as IFI44, IFI27, or myxovirus resistance-1 (Mx1) [10,21]. The results of the present study showed that the expression of CXCL10 correlated with the level of STAT1 phosphorylation. However, the expression of IFI44 did not correlate with the level of STAT1 phosphorylation (Fig. 3B). The lack of correlation between the expression of IFI44 and the level of STAT1 phosphorylation might be attributed to the limited number of patients enrolled.
Hepatitis C virus infection is associated with the development of various extrahepatic manifestations [3]. They might partly stem from the HCV infection to various types of PBMCs, such as monocytes, dendritic cells, and lymphocytes [11] leading to the activation of type I IFN response in these cells [30]. Eradication of virus with DAA is associated with a reduction not only in liver-related events of HCV infection, but also in HCV-related extrahepatic manifestations [31]. A recent report showed that IFNL4 genotype (previously known as IL-28B genotype) influences the baseline ISG expression level in the PBMCs of HCV-infected patients suggesting a functional link between the IFNL4 genetic variation and immune response in cells of extrahepatic origin [32]. Given that the HCV-infected patients with cryoglobulinemic vasculitis [33] and metabolic extrahepatic manifestations [34] have higher frequency of IL-28B T/T genotype, which is proven to cause high intrahepatic baseline ISG levels [2,7,9], a decreased expression of extrahepatic ISGs by DAAs observed in the present study might partly explain the alleviation of extrahepatic manifestations after DAA treatment.
The present study had some limitations: (1) the expression of ISGs was evaluated in unstimulated PBMC, which might not reflect the true activity of IFN in the liver, (2) the analyses were performed using unsorted PBMCs, which might lead to confusion during data analyses, and (3) the relatively small number of patients enrolled limited the analyses.
The present study demonstrated that the pretreatment activation of IFN-β response is normalized after DAA treatment. The results suggest that the decreased expression of ISGs might be one of the mechanisms of DAA-induced alleviation of extrahepatic manifestations during HCV infection.
Notes
Author contributions
P.S.S., E.B.L, and S.K.Y. designed and carried out the study; P.S.S., E.B.L, D.J.P., A.L., J.W.J., S.H.B., J.Y.C., and S.K.Y. analyzed the data; P.S.S. and S.K.Y. wrote the paper.
Acknowledgements
The present study was partly supported by the Global Hightech Biomedicine Technology Development Program of the National Research Foundation (NRF) and Korea Health Industry Development Institute (KHIDI) funded by the Korean government (MSIP & MOHW) (No. 2015M3D6A1065146). This research was also supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2017R1D1A1B03033718).
Notes
Conflict of Interest
The authors declare no financial conflict of interest.
Abbreviations
ALT
alanine aminotransferase
cDNA
complementary DNA
CXCL10
C-X-C motif chemokine ligand 10
DAA
direct-acting antiviral
GAPDH
glyceraldehyde 3-phosphate dehydrogenase
HCV
hepatitis C virus
HRP
horseradish peroxidase
IFI44
interferon-induced protein 44
IFN
interferon
ISG
interferonstimulated gene
JAK
Janus kinase
MAVS
mitochondrial antiviral signaling protein
Mx1
myxovirus resistance-1
NS5A
non-structural 5A
PBMC
peripheral blood mononuclear cell
PCR
polymerase chain reaction
PY-STAT1
tyrosine-phosphorylated STAT1
RAS
resistance-associated substitution
RIPA
radioimmunoprecipitation assay
SOF
sofosbuvir
STAT1
signal transducer and activator of transcription 1
SVR
sustained virologic response
TRIF
Toll/IL-1 receptor domain-containing adaptor inducing IFN-β
References
Article information Continued
Notes
Study Highlights
Hepatitis C virus (HCV) infection stimulates the induction of extrahepatic type I interferons (IFN), leading to the upregulation of interferon-stimulated genes. Pretreatment activation of extrahepatic type I IFN response is normalized after treatment of HCV infection with direct acting antivirals (DAAs). Our study suggests that the decreased type I IFN response by the clearance of HCV might contribute to DAA-induced alleviation of extrahepatic manifestation of chronic HCV infection.

