Virologic response is not durable after adefovir discontinuation in lamivudine-resistant chronic hepatitis B patients
Article information
Abstract
Background/Aims
We investigated the durability of the biochemical and virologic responses after adefovir (ADV) discontinuation in lamivudine-resistant (LMV-R) chronic hepatitis B (CHB) patients, and the outcomes of ADV discontinuation compared to that of ADV maintenance.
Methods
The indication for ADV treatment cessation was an undetectable level of hepatitis B virus (HBV) DNA documented on two occasions at least 6 months apart. All patients received additional ADV for at least 12 months after the confirmation of undetectable HBV DNA (Cobas TaqMan PCR assay, <70 copies/mL). Of 36 patients who had a sufficient ADV therapeutic effect, 19 discontinued ADV treatment, while the others maintained it. A virologic rebound was arbitrarily defined as the redetection of HBV DNA at a level higher than 105 copies/mL.
Results
In the ADV discontinuation group, ADV treatment and additional therapy were administered for medians of 33 months (range, 12-47 months) and 18 months, respectively. The patients were followed for a median of 12 months (range, 3-30 months) after ADV cessation. During that period, 18 of 19 patients (95%) experienced viral relapse. Viral rebound was observed in six patients (32%). However, 12 of 18 patients (67%) exhibited serum HBV DNA levels of less than 105 copies/mL. Biochemical relapses were observed in four of the six patients with viral rebound. In the ADV maintenance group, patients were treated for a median of 53 months (range, 31-85 months), and 9 patients (53%) experienced viral breakthrough.
Conclusions
During short-term follow-up after ADV discontinuation, most patients (95%) exhibited viral relapse, whereas and viral breakthrough occurred in about half of patients (53%) maintained on ADV therapy. Therefore, the durability of virologic response after ADV discontinuation in LMV-R patients was unsatisfactory. In addition, and viral breakthrough was not infrequent in the ADV continuation group.
INTRODUCTION
Adefovir dipivoxil (ADV) is an antiviral agent that is effective against wild-type hepatitis B virus (HBV) as well as lamivudine-resistant (LMV-R) HBV. Large-scale clinical research in treatment-naïve chronic hepatitis B (CHB) patients has shown ADV to be effective from biochemical, virological, and histological perspectives.1-3 After treatment with ADV, durability is rarely reported. However, in some patients, HBeAg seroconversion is reportedly sustained 1 year after ADV therapy,4 and among HBeAg-negative CHB patients who had had 5 years of effective ADV therapy that was then discontinued, 66% had a sustained biochemical and virological response.5
Several authors have observed biochemical and virological benefits in LMV-R CHB patients treated with ADV either alone or added to ongoing lamivudine therapy.6-8 A recent report showed that a virological response was achieved in 55% of patients treated with adefovir and that a biochemical response was achieved in 83%.9 However, whether the viral response is sustained after ADV discontinuation in LMV-R CHB patients receiving ADV is unknown. In this study, we investigated the durability of the biochemical and virological responses after discontinuation of ADV in LMV-R CHB patients, and compared the responses in patients who discontinued ADV with patients who continued ADV.
PATIENTS AND METHODS
Patients
A total of 120 patients were treated with ADV monotherapy for lamivudine resistance. LMV-R CHB was defined as viral breakthrough or genotypic resistance of lamivudine.2 We retrospectively analyzed their virologic and biochemical responses. Among them, 36 patients showed a virological response, and subsequently discontinued ADV. We then established consistent criteria as follows: the enrollment criterion for HBeAg-positive patients was confirmed HBeAg seroconversion with undetectable levels of HBV DNA documented on two separate occasions at least 6 months apart, while the criterion for HBeAg-negative patients was undetectable HBV DNA, documented on two separate occasions at least 6 months apart. All patients received additional ADV for at least 12 months after confirmation of undetectable levels of HBV DNA according to the Asian-Pacific consensus statement on the management of CHB.10 Nineteen patients who satisfied the criteria were enrolled as ADV discontinvation group and followed for more than 3 months after ADV discontinuation. Seventeen patients who also satisfied the criteria continued ADV monotherapy.
Methods
A liver biochemical test, quantitative HBV DNA, HBeAg, and anti-HBe tests were carried out upon discontinuation of ADV. Furthermore, these tests were repeated every 3 months afterwards, and the therapeutic effect of ADV was examined during this follow-up period. Serum HBV DNA was measured using the TaqMan polymerase chain reaction assay (COBAS TaqMan, Roche Molecular Systems; low detection limit was less than 70 copies/mL), and HBeAg and anti-HBe were measured using a radioimmunoassay kit (HBeAg/Ab IRMA kit, Beijing North Institute of Biological Technology). Genotypic resistance was analyzed in some patients with suspected LMV-R CHB and performed using the restriction fragment mass polymorphism method (Green Cross Reference Lab., Yongin, Korea) as previously described.11
Definitions
"Viral relapse" was defined as HBV DNA detection by the PCR method after achieving undetectability of HBV DNA, and "viral rebound" was defined as HBV DNA detection at more than 105 copies/mL during the follow-up period after ADV discontinuation. "Viral breakthrough" was defined as a greater than or equal to 1 log10 increase in the serum HBV DNA level from nadir on two consecutive occasions after an initial virological response.2 "Decompensation" was defined as the development of ascites, jaundice, bleeding varices, or hepatic encephalopathy. "Biochemical relapse" was defined as an elevated alanine aminotransferase (ALT) value above the upper normal limit after achieving ALT normalization, and "liver cirrhosis" was based on histological or ultrasonographic findings of a blunted, nodular liver surface accompanied by splenomegaly (>10 cm) with a low platelet count (<100,000/mm3).
Statistical analysis
For categorical variables, differences between groups were calculated using Fisher's exact test. For continuous variables, differences were calculated by the t-test. Logistic regression analysis was used to derive the odds ratio and 95% confidence interval (CI) for the viral rebound's influence on HBV DNA of >104 copies/mL. The cumulative probability of ADV-resistant mutations was estimated by Kaplan-Meyer analysis. A p-value of less than 0.05 was considered to indicate statistical significance. All analyses were performed using the SPSS software (ver. 12.0 for Windows; SPSS, Inc., Chicago, IL, USA).
RESULTS
Baseline characteristics
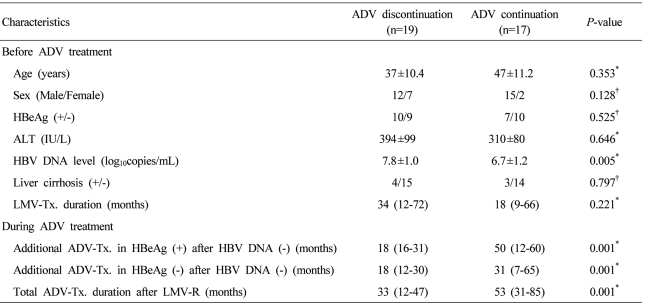
Baseline characteristics of the 36 patients are summarized according to ADV continuation or discontinuation in Table 1. In ADV discontinuation group, before ADV treatment the mean age of enrolled patients was 37 (SD±10.4) years, and four of them showed compensated liver cirrhosis with a Child-Pugh score of 5. In ADV continuation group, the mean age was 47 (SD±11.2), and 3 patients had compensated liver cirrhosis. In ADV continuation group baseline HBV DNA level was lower compared with that in ADV discontinuation group (P=0.005). However, other factors showed no difference between two groups before ADV treatment. During ADV treatment, ADV continuation group showed more longer treatment period than ADV discontinuation group (P=0.001).
In ADV discontinuation group, ten patients were HBeAg positive, and the mean serum ALT and HBV DNA levels were, respectively, 394 (SD±99) IU/L and 8.3 (SD±0.2) log10 copies/mL before ADV treatment. The patients were treated with ADV for a median of 33 (range, 12-47) months, and after the treatment, additional therapy was administered for a median of 18 months. In ADV continuation group, they continuously received ADV monotherapy for a median of 53 months (range, 31-85).
Durability of virological and biochemical response after ADV discontinuation
During the follow-up period after ADV treatment, 18 of the 19 patients (95%) experienced viral relapse after ADV discontinuation. In addition, six of the 18 patients (33%) showed viral rebound as previously defined and their serum HBV DNA levels continued to increase with time and accompanied by ALT elevation. One patient in the viral rebound group experienced HBeAg reversion, but decompensation was not observed. Patients who experienced biochemical relapse were retreated with antiviral agents. Three patients were retreated with ADV monotherapy because they showed a good response (Table 2). The virologic and biochemical responses after ADV discontinuation were not significantly different between HBeAg positive and negative patients.
Fluctuation of HBV DNA levels during follow-up period after ADV discontinuation
The change in the HBV DNA level for each patient after ADV discontinuation is shown in Figure 1. All except one patient experienced viral relapse at 3 months after discontinuation. The numbers of patients in accordance with HBV DNA levels of relapse at 3 months were as follows: four at >105 copies/mL, four from 105 to 104 copies/mL, 10 at <104 copies/mL, and one below the detection limit. Although HBV DNA levels showed fluctuation during the follow-up period, the numbers of patients in accordance with HBV DNA levels of relapse at 12 months of discontinuation or at the endpoint of follow-up were as follows: four at >105 copies/mL, three from 105 to 104 copies/mL, 11 at <104 copies/mL, and one below the detection limit. Viral rebound was observed in six patients (33%), but the remaining 12 (67%) showed a serum HBV DNA level of less than 105 copies/mL. In the viral rebound group, some showed biochemical relapses, HBeAg reversion, and even ALT flare-up.
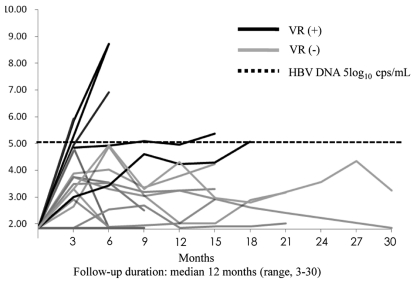
Graph showing changes in HBV DNA levels during the follow-up period after ADV discontinuation. The thick solid lines illustrate the change in the HBV DNA level of patients with viral rebound, and the thin solid lines illustrate viral relapse without viral rebound. Viral rebound was defined as HBV DNA being detected at more than 105 copies/mL during the follow-up period after ADV discontinuation. The dotted line indicates 5 log10 copies/mL. Viral rebound (VR) was defined as HBV DNA detection at more than 105 copies/mL during the follow-up period after ADV discontinuation.
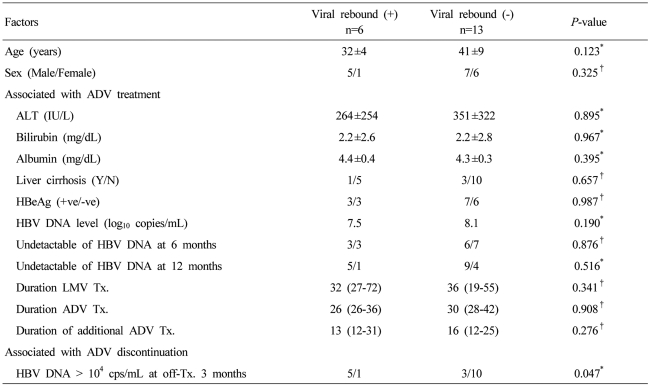
Risk factors for viral rebound after ADV discontinuation
We analyzed various factors for a possible association with viral rebound, including age, sex, ALT level, cirrhosis, baseline HBV DNA level, viral response to ADV treatment, and the additional duration of ADV treatment. The only factor significantly associated with viral rebound was an HBV DNA level of more than 104 copies/mL 3 months after ADV discontinuation (Table 3). Additionally, logistic regression analysis was used to identify influence of each factor on viral rebound. An HBV DNA level of more than 104 copies/mL 3 months after ADV discontinuation showed a significant influence (odds ratio, 13.333; 95% CI, 1.048-169.557; P=0.046).
Viral breakthrough in ADV continuation group
We analyzed viral breakthrough in ADV continuation group. Viral breakthrough was observed after 38 months in ADV continuation group. They received ADV monotherapy for a median of 53 months (range, 31-85). During ADV treatment 9 patients (53%) experienced a viral breakthrough. The patients experiencing a viral breakthrough did not show decompensation and all of them received rescue therapy with entecavir.
DISCUSSION
It is not clear when to stop nucleoside/nucleotide analogue (NA) treatment; ideally, however, the drug should be discontinued after HBsAg loss during NA treatment in HBeAg-positive and -negative patients.12 In an HBsAg loss state, HBV DNA is not detectable in serum or is detectable only at low levels in liver. This state is associated with an improvement in outcomes, including a reduced risk of cirrhosis and hepatocellular carcinoma (HCC).13 However, HBsAg loss is rarely experienced during NA treatment.14,15 In many countries, the current practice guidelines for treatment end points suggest that treatment can be discontinued after seroconversion to anti-HBe, achievement of a decline in HBV DNA to non-detection by PCR, and a return of ALT to normal levels. If needed, additional therapy of more than 6 to 12 months can be considered.2,10,12 In the present study, we applied "strict" enrollment criteria: ALT normalization and undetectable HBV DNA by PCR, sufficient additional therapy (more than 12 months), and, in HBeAg-positive CHB patients, HBeAg seroconversion.10
Several studies on the durability of LMV in CHB patients have been reported. A Western study showed a 2-year cumulative rate of recurrence of approximately 50%.16 An Asian study reported that 49.2% of CHB patients with HBeAg seroconversion and short additional therapy experienced recurrence, and risk factors were the pretreatment HBV DNA level and the period of additional therapy.17 However, few data are available concerning the durability of ADV in treatment-naïve CHB patients. One study reported that 90% of 76 patients had sustained HBeAg seroconversion 1 year after ADV discontinuation.18 Additionally, a sustained response rate following the discontinuation of ADV after 4 or 5 years of effective ADV treatment has been reported in up to 66% of ADV treatment-naïve patients. However, whether the viral response is sustained after ADV discontinuation in LMV-R CHB patients receiving ADV is unknown.5
The aim of this study was to investigate the durability of the virological and biochemical responses after ADV discontinuation in LMV-R patients, to analyze the factors associated with viral rebound, and to find out the benefit of ADV discontinuation compared to ADV continuation treatment. During the follow-up period (a median of 11 months) in ADV discontinuation, almost all patients showed viral relapse; that is, they showed detectable levels of HBV DNA by PCR. During the followup period, 12 patients (67%) showed a low serum HBV DNA level (fewer than 105 copies/mL), and biochemical relapses were observed four patients (21%). These results are similar to those of an earlier report on ADV treatment-naïve CHB patients.5
The mechanism by which a high HBV DNA level causes the progression of liver disease is not fully understood. The role of HBV DNA level was recently reported in a natural history study. The REVEAL study showed that a baseline level of HBV DNA of >100,000 copies/mL was a strong predictor for HCC development compared with a baseline level of <100,000 copies/mL.19 Zacharakis et al showed in a prospective cohort study showed that an HBV DNA level of >10,000 copies/mL and a normal ALT at baseline and at follow-up were independent predictors20 for liver disease progression in inactive carrier state patients. In other studies, after HBeAg seroconversion, most patients showed sustained normal serum ALT, low serum HBV DNA (fewer than 105 copies/mL), and minimal histological changes.21-23 In the present study, although we used "strict" discontinuation criteria, 63% showed sustained normal ALT and low HBV DNA levels (less than 105 copies/mL) during the follow-up period.
As for continuation of the ADV treatment, our study did not show a satisfactory result in ADV continuation group although the patient number is small. During a median of 53 months (range, 31-85), totally 9 patients (53%) experienced a viral breakthrough. Although the randomized prospective study, 17 patients enrolled in ADV continuation group showed a good initial response to ADV treatment, but about half of them finally experienced viral breakthrough. Thus, the decision as to when to stop HBV treatment needs further consideration.
In past studies of other NA treatments, the durability of and response to NA treatment were associated with the pretreatment HBV DNA level and ALT value, age, and additional treatment period.17,24,25 In our study, we analyzed multiple factors, including the above factors, but the only factor associated with viral rebound and biochemical relapse was an HBV-DNA level of more than 104 copies/mL 3 months after ADV discontinuation. We believe that this shows that close follow-up is very important in the early period after NA discontinuation, and we assume that we did not find other risk factors associated with relapse because we analyzed such a small number of patients.
Our study has some limitations because of its retrospective nature, although we used strict enrollment criteria according to APASL guidelines. First, the sample size was small, and because patients were enrolled by data selection, selection bias was present. Second, genotype mutation analysis of lamivudine was not performed in some patients, so we could not investigate risk factors according to the genotype mutation pattern. Third, ADV monotherapy in LMV-R CHB patients is no longer recommended according to the current guidelines, so the results of this study will not affect the decision of the current treatment direction. However, despite these limitations, this study offers a current guideline regarding the discontinuation of antiviral agents when they are not very effective and when longer treatment duration may be needed in LMV-R CHB patients.
In conclusion, during a short-term follow-up of ADV discontinuation, most patients showed viral relapse; approximately two-thirds of patients with viral relapse maintained a normal ALT level and an HBV-DNA level of fewer than 105 copies/mL, and one-third of patients experienced viral rebound and re-treatment with antiviral agents. In ADV maintenance about half of patients (53%) showed viral breakthrough. Therefore, antiviral response was not durable after ADV discontinuation in LMV-R patients was unsatisfactory, and the benefit was modest compared to ADV maintenance. In LMV-R patients, antiviral treatment with more potent drugs and longer duration is recommended.
Abbreviations
ADV
adefovir
LMV-R
lamivudine-resistanct
CHB
chronic hepatitis B
ALT
aminotransferase
CI
confidence interval
VR
viral rebound
NA
nucleoside/nucleotide analogue