Personalized management of cirrhosis by non-invasive tests of liver fibrosis
Article information
Abstract
Owing to the high prevalence of various chronic liver diseases, cirrhosis is one of the leading causes of morbidity and mortality worldwide. In recent years, the development of non-invasive tests of fibrosis allows accurate diagnosis of cirrhosis and reduces the need for liver biopsy. In this review, we discuss the application of these non-invasive tests beyond the diagnosis of cirrhosis. In particular, their role in the selection of patients for hepatocellular carcinoma surveillance and varices screening is highlighted.
INTRODUCTION
Despite geographical differences, chronic liver diseases are highly prevalent worldwide. It is estimated that at least 350 million and 120 million people globally are chronically infected with hepatitis B virus (HBV) and hepatitis C virus, respectively.12 Non-alcoholic fatty liver disease (NAFLD) affects 15-40% of the general population and is particularly prevalent in patients with diabetes and obesity.345 Alcoholic liver disease affects both developed and developing countries and may account for up to 9.2% of all disability-adjusted life years in some regions.6 Although the etiologies are different, chronic liver disease leads to liver injury, progressive liver fibrosis, and finally to the stage of cirrhosis and liver decompensation. As a result, cirrhosis remains the twelfth global leading cause of death in 2010.7
The diagnosis of cirrhosis is not as simple as it seems. Evidently, the diagnosis is straightforward when a patient has already developed clinical manifestations of portal hypertension such as ascites, varices and hypersplenism. Nonetheless, these signs are absent in patients with early cirrhosis, and the radiological features of early cirrhosis are subtle and unreliable.8 Liver biopsy is traditionally the gold standard for the diagnosis of cirrhosis. However, it is an invasive procedure with a small risk of bleeding. The poor patient acceptance and the risk of sampling error (understaging due to inadequate sample) further limit the widespread application of liver biopsy.
In recent years, the development and application of non-invasive tests of liver fibrosis have revolutionized hepatology practice. Numerous studies have confirmed the accuracy of these tests in fibrosis staging and the diagnosis of cirrhosis. In general, the tests have high negative predictive value in excluding advanced fibrosis and cirrhosis and have been recommended by the European Association for the Study of the Liver as initial assessment in patients with various liver diseases.9
In addition, cirrhosis is not one single disease but encompasses a broad spectrum of clinical condition ranging from compensated disease to decompensated disease. As the disease progresses, various complications of portal hypertension may develop. The development of hepatocellular carcinoma (HCC) further drifts the clinical course and leads to major morbidity and mortality. Therefore, one important part of the management of cirrhosis is to identify and treat major complications early. In this review, we first provide an overview on non-invasive tests of liver fibrosis. Since the diagnosis of cirrhosis is only the first step in the management of cirrhosis, we further discuss the potential application of these tests in the risk stratification of cirrhosis and prediction of cirrhotic complications.
NON-INVASIVE TESTS OF LIVER FIBROSIS
Non-invasive tests of liver fibrosis have been a hot research area in the past decade. At the beginning, the main focus was to reduce the burden of liver biopsy by confidently identifying patients who are very unlikely to have significant fibrosis on one hand and those who are very likely to have advanced fibrosis or cirrhosis on the other. Treatment decisions can then be made accordingly, and patients in the middle (gray zone cases) may undergo liver biopsy or be observed over time. In general, non-invasive tests of liver fibrosis can be divided into serum tests and physical measurements.
Serum tests
The advantages of serum tests include high applicability (successful measurements can be made in most cases) and relatively simple logistics. Doctors may obtain blood samples at their clinics and send them to designated laboratories even for more specific biomarkers. Serum tests can be divided into class I biomarkers and class II biomarkers. Class I biomarkers specifically measure the activity of fibrogenesis or fibrinolysis. In contrast, class II biomarkers do not measure fibrosis directly but represent parameters that correlate with fibrosis. For example, aspartate aminotransferase (AST) is a marker of hepatic necroinflammation and not fibrosis. However, patients with fibrosis and cirrhosis often have increased AST levels. Although class I biomarkers are expected to directly reflect fibrosis and be more accurate than class II biomarkers, this has not been consistently demonstrated in prospective studies.
In any case, at present there is no single marker that can adequately reflect fibrosis. Therefore, in most situations several biomarkers or a combination of biomarkers and other clinical features are used. Some of the combined panels such as FibroTest, FibroMeter and enhanced liver fibrosis (ELF) score have been commercialized. It should be noted that such combined tests are modeled against liver histology, which is an imperfect reference standard. In other words, even if the models can 100% faithfully reflect liver histology, the accuracy of liver histology to diagnose fibrosis and cirrhosis will be the ceiling of accuracy of the new models.10
Some of the class II biomarkers are more generic. Examples include the AST-to-alanine aminotransferase (ALT) ratio and the AST-to-platelet ratio index (APRI). In other cases, owing to the pathophysiology of different liver diseases, the class II biomarkers are more disease-specific. For instance, metabolic factors are overrepresented in the NAFLD fibrosis score, which should only be applied in patients with NAFLD.1112
Physical measurements
The other main class of non-invasive tests of liver fibrosis relies on physical measurement of liver stiffness and elasticity. Although the cutoffs of the measurements are determined with reference to histology, these tests are not modeled against histology and theoretically may achieve better prediction than histology. In fact, studies in patients with chronic viral hepatitis suggest that transient elastography may be better than histology in predicting overall mortality.1314
Transient elastography by FibroScan (Echosens, Paris, France) is currently the most commonly used method to measure liver stiffness.15 It estimates liver fibrosis by measuring the velocity of a shear wave in the liver parenchyma. This is based on the physical principle that waves travel faster in a stiffer medium. The main advantage of transient elastography is the ease of use and high reproducibility.16 Compared with serum tests, transient elastography is less applicable in obese patients, although the new XL probe partially compensates for that.17 Liver stiffness is also affected by high ALT level, hepatic congestion, food intake and amyloidosis. Because the correlation between liver stiffness and fibrosis is a generic phenomenon, the technique can be applied to patients with different liver diseases, though the appropriate cutoffs are higher in patients with alcoholic liver disease.18
Newer techniques such as acoustic radiation force impulse and shear-wave elastography allow simultaneous visualization of the liver parenchyma and measurement of liver elasticity.1920 This can thus combine HCC surveillance and liver fibrosis assessment in a single examination. Magnetic resonance elastography appears to be highly accurate and is not affected by obesity, but machine availability and the costs of examination can be prohibitive.21 These new techniques also have not been as extensively validated as transient elastography.
For simplicity, doctors usually adopt one or more recommended cutoff values in the interpretation of any of the non-invasive tests above. In reality, however, the more extreme the values of the non-invasive tests are, the more we are confident in whether a patient has fibrosis/cirrhosis or not. For example, a probability-based interpretation of liver stiffness measurements (LSM) has been proposed.22 Based on the exact LSM, the probability of a patient having different fibrosis stages can be determined. The complexity of this approach limits its use in real life practice. Nonetheless, as a patient is more likely to have cirrhosis and advanced cirrhosis when he has more extreme non-invasive tests results, this forms the basis of extending the tests to stratify the risk and predict complications in cirrhotic patients.
HEPATOCELLULAR CARCINOMA
Current recommendations and unmet need
HCC is one of the most important complications in patients with chronic liver diseases.23 HCC surveillance is an indispensable part of the management of liver patients. The current Asian Pacific guidelines recommend 6-monthly trans-abdominal ultrasonography and serum alpha-fetoprotein (AFP) testing for HCC surveillance.24 Despite the fact that AFP has been widely adopted for decades, it has been criticized as neither sensitive nor specific.25 Hence the latest American guidelines stopped recommending the use of AFP in the surveillance program.26 Nonetheless, a recent study demonstrated a satisfactory sensitivity and specificity of AFP for HCC in CHB patients received antiviral therapy.27 Therefore some experts believed that the calls for abandoning the monitoring of AFP levels might be premature, especially given the already low HCC surveillance rate in developing countries and at primary care settings.28
HCC surveillance improves the prognosis of patients by identifying tumors of smaller sizes, fewer numbers of tumors, and longer overall survival.29 Unfortunately, the 5-year mortality rate was still close to 40% despite the regular HCC surveillance. This observation points towards the fact that the current HCC surveillance recommendation is still far from satisfactory.
The key components of an optimal surveillance program include accurate risk stratification and reliable surveillance tools. Hence there is a need for accurate HCC risk prediction to assist prognostication as well as decision on the need for HCC surveillance.
Non-invasive tests and HCC risk
Liver stiffness measurements
Various non-invasive tests of liver fibrosis have been tested to predict the risk of HCC. Among them LSM with transient elastography is the most widely-studied. A dose-response relationship between LSM and HCC risk was demonstrated in patients with either chronic hepatitis B (CHB) or chronic hepatitis C (CHC).3031 In a prospective cohort of study of 1,130 Korean CHB patients, the hazard ratios (HRs) of developing HCC were 3.1, 4.7, 5.6, and 6.6 in patients with LSM at 8.1-13.0 kPa, 13.1-18.0 kPa, 18.1-23.0 kPa, and >23.0 kPa, respectively, when compared to those with LSM LSM ≤ 8.0 kPa.30 A similar relationship was demonstrated in another prospective cohort of 866 Japanese CHC patients that the HRs of HCC were 17, 21, 26, and 46 in patients with LSM at 10.1-15.0 kPa, 15.1-20.0 kPa, 20.1-25.0 kPa, and >25.0 kPa, respectively, with reference to those with LSM ≤ 10.0 kPa.31 These findings implied that LSM is a useful parameter to estimate HCC risk in patients with chronic liver disease across the etiologies despite different carcinogenetic mechanisms.
Serum tests
FibroTest is one of most popular serum-based non-invasive tests for liver fibrosis.32 FibroTest was recently shown to be as good as LSM to stage patients with viral hepatitis into seven categories such that it could accurately predict severe complications, of which most were HCC.3334 In a multicenter study of 1,312 French patients with chronic hepatitis B, using the predetermined stages of FibroTest at ≤0.27, >0.27-0.48, >0.48-0.58, >0.58-0.74, >0.74-0.85 and >0.85-1, HCC developed in 0.4%, 0.7%, 4.4%, 13.6%, 11.1% and 44.7% of patients, respectively.34
Another serum-based non-invasive test, enhanced liver fibrosis (ELF) test has been increasingly used to assess liver fibrosis by its or in combination with LSM in CHB patients.35 In a Korean study, ELF was found superior to LSM, histologic fibrosis stage, or age-spleen-platelet ratio to predict liver-related events, again HCC accounting for most of the events.36 Compared with patients with high ELF ≥10.40, patients of low (<8.10) and intermediate (8.10-10.39) ELF scores had much lower risk of liver-related events (adjusted HR 0.045 and 0.239 respectively).36
HCC risk score based on non-invasive tests
The risk factors of HCC in patients with chronic hepatitis B are well known, and various groups have derived HCC risk scores based on the factors.37 Since cirrhosis is the single most important risk factor for HCC development, non-invasive tests of fibrosis may improve prediction by not only diagnosing cirrhosis more accurately but also reflecting the severity of cirrhosis. To further consolidate the important role of LSM on HCC risk prediction, it was incorporated into a risk score for HBV-related HCC, called LSM-HCC score, together with three other important clinical parameters namely age, serum albumin and HBV DNA level.38 LSM has replaced clinical cirrhosis, the heavily weighed component in the original CU-HCC score,39 in order to provide more objective and accurate diagnosis of cirrhosis (Table 1). This new LSM-HCC score excludes future HCC with high negative predictive value (99.4-100%) at 5 years.38
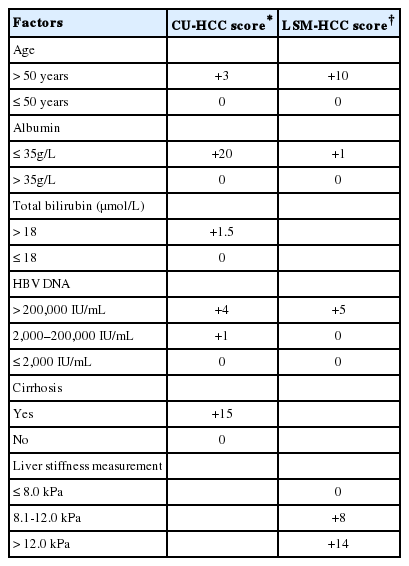
CU-HCC score vs. LSM-HCC score [Modified from reference Wong VW et al. JCO 2010 & Wong GL et al. J Hep 2014]
LSM has also been integrated with age, gender, HBV DNA level into a regression formula to predict HCC with good accuracy.40 Adding LSM to another commonly used HCC risk score REACH-B score achieved a higher accuracy to predict liver-related events (of which 60% were HCC) when compared to REACH-B score alone (area under the receiver-operating characteristics curve 0.81 vs. 0.63).41 Although serum-based non-invasive tests are probably equally popular for assessing liver fibrosis, so far no HCC risk score has been developed based on these tests.
VARICES
Current recommendations and unmet need
Portal hypertension is one of the most lethal complications of chronic liver disease.42 Thus, it is vital to identify patients with varices and institute primary prophylaxis. According to the American Association for the Study of Liver Diseases, all diagnosed patients with cirrhosis should undergo screening esophagogastroduodenoscopy (EGD).43 For patients without varices during screening, surveillance EGD should be done after two to three years. For those with small varices, surveillance EGD is recommended after one to two years. Annual EGD is warranted in patients with liver decompensation.43
However, EGD is unpleasant. Some patients at risk would never agree to undergo EGD because of the perceived discomfort. On the other hand, many other patients go through the procedure with negative findings. This may cause unnecessary suffering and increase health costs. The use of thrombocytopenia and splenomegaly has been proposed to identify patients with portal hypertension and varices, but the accuracy remains limited.44
Non-invasive tests and portal hypertension
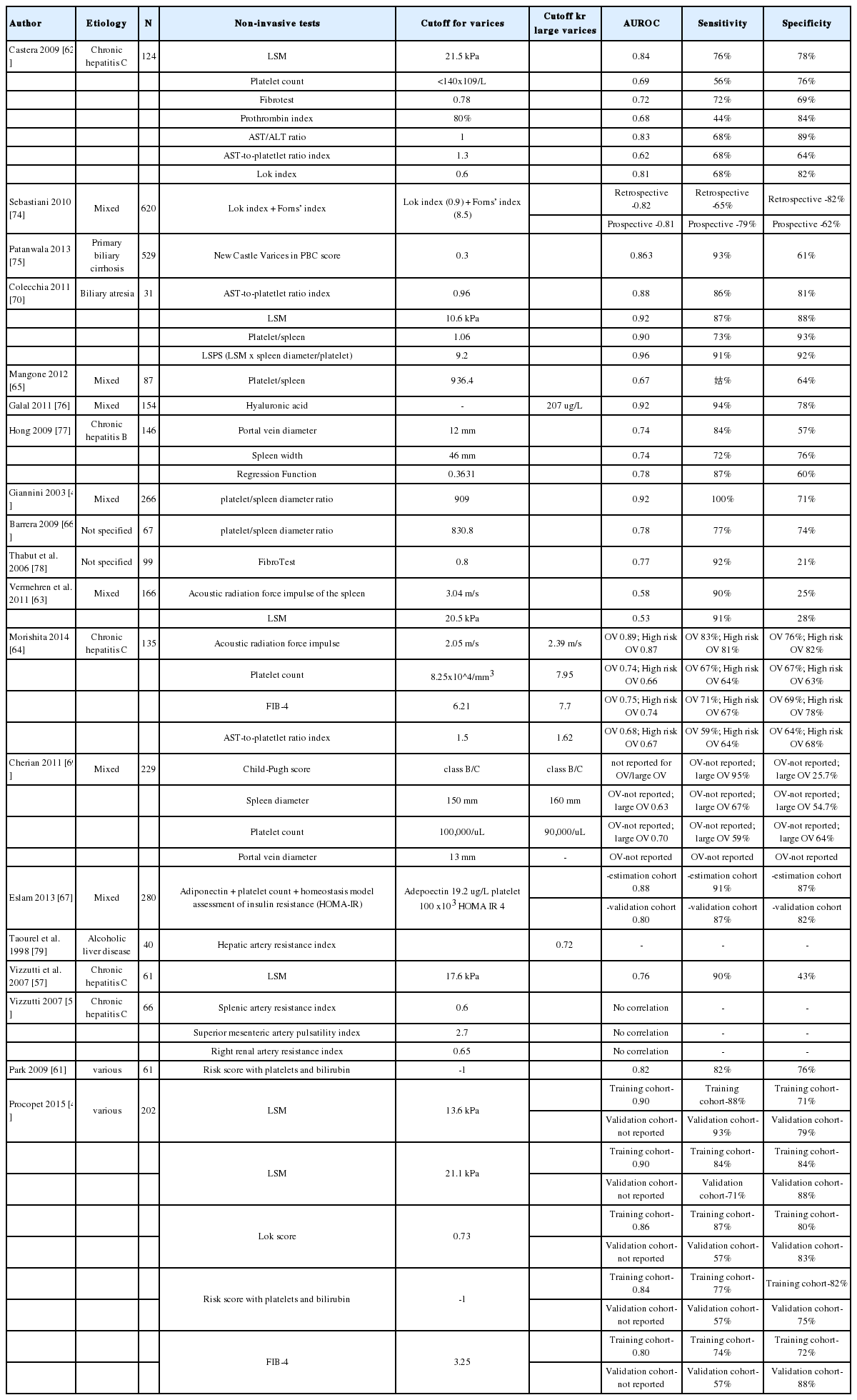
Ascites, hepatic encepalopathy, and variceal bleeding are some of the complications of liver cirrhosis. The development of these events is due to portal hypertension. The gold standard for detecting portal hypertension is through the measurement of hepatic venous pressure gradient (HVPG). However, it is invasive and not readily available. Therefore, a number of studies have evaluated the use of non-invasive tests to predict portal hypertension (Table 2). One of the most commonly used is transient elastography. Given that fibrosis is a major contributor to elevated hepatic resistance (measured by HVPG), the role of transient elastography as a surrogate measure of HVPG has been studied.45 Several cutoff levels have been proposed ranging from 8.7 kPa (HVPG >6 mmHg) to 34.9 kPa (HVPG >10 mmHg) depending on the population being tested.4647 It has a sensitivity of 63-100% and a specificity of 41-96% to determine portal hypertension.4647484950 In addition, in a study among cirrhotics, an LSM of >21.1 kPa is as accurate as HVPG in identifying patients at risk portal hypertensive complications.48
Splenomegaly and hypersplenism are features of portal hypertension. Indeed, spleen stiffness (54 kPa) can predict survival free complications among HCV-related cirrhotic patients.4851 However, it should be highlighted that transient elastography measures a core of tissue that is 4 cm in length; it has not been optimized for the measurement of spleen stiffness. In that regard, other physical measurements such as acoustic radiation force impulse may be more appropriate.52
Furthermore, the use of duplex doppler ultrasonography has been an attractive non-invasive test to determine portal hypertension by assessing the vascular anatomy and its hemodynamics. Parameters such as portal blood flow and velocity, resistive and pulsatility indices have also been explored to predict HVPG although with conflicting results. In a study of cirrhotic patients with different etiologies, portal vein velocity was found to correlate with HVPG, but the findings have not been confirmed by all studies.535455 Other examinations of the hepatic, splenic, superior mesenteric arteries were not proven to be correlated with HVPG.535455565758 Interestingly, some ultrasound parameters such as hepatic waveforms and damping index may be used to determine the response to medications to decrease portal pressure.5960 In contrast, portal vein velocity is not useful for the monitoring of response to terlipressin.5458 These conflicting results may be due to differences in techniques used and heterogeneity of populations being studied such as of etiology of cirrhosis, Child Pugh score, and use of medications.
A Korean group further developed a simple risk score comprising bilirubin and platelets to predict HVPG.61 It has a sensitivity of up to 88% and specificity of up to 86% in predicting HVPG >10 mmHg. Other tests like the Lok index and FIB-4 had fair accuracy in predicting HVPG.4961
Non-invasive tests and varices
As mentioned above, EGD should be performed for varices screening in cirrhotic patients, but the procedure is unpleasant and often omitted. Since non-invasive tests of fibrosis can reflect portal hypertension, it is logical to consider their application in selecting patients for EGD. LSM has the highest accuracy with a sensitivity of 76-91% and specificity varies of 28-88% to detect esophageal varices (Table 3).50516263 Some authors used acoustic radiation force impulse of spleen and liver with good accuracy (ARFI spleen sensitivity 90% and specificity 76%; ARFI liver sensitivity 83% and specificity 76%).6364 Several studies have evaluated the platelet-to-spleen ratio with an adequate sensitivity of 64-100% and specificity 64-93%.44516566 Furthermore, to predict high risk varices or variceal bleeding, ARFI and Child-Pugh class were noted to correlate with these end points.6467 Other blood tests that were determined to predict varices were platelet count (sensitivity 56-76%; specificity 64-88%) and AST-to-platelet Index (sensitivity 59-86%; specificity 64-81%).6264686970 Among the doppler ultrasound parameters only hepatic artery resistive index was found to be associated with presence of varices.5557
In the recent Baveno VI guideline, some of these non-invasive tests were already incorporated to stratify patients.71 It recommends that patients with LSM <20 kPa and platelet count >150,000 have very low risk of having large varices; screening EGD can be avoided. However, these very low risk patients should undergo annual transient elastography and platelet count.
CONCLUSIONS
Non-invasive tests of liver fibrosis have revolutionized the management of chronic liver diseases. Compared with routine clinical assessments, the non-invasive tests allow more confident diagnosis of cirrhosis and can also reflect the severity of cirrhosis and/or portal hypertension. They can therefore be used to select patients for HCC surveillance and varices screening.
That said, there are still a number of questions regarding the use of the non-invasive tests. The interval of testing is currently undefined, and it is unclear to what extent the changes in the non-invasive tests reflect fibrosis progression. In addition, patients with treated viral hepatitis often have reduced liver stiffness and improved serum tests of fibrosis.72 While some of these patients indeed have reduced fibrosis, it is unclear when this indicates regression of cirrhosis and whether the interpretation is the same as in untreated patients. Similarly, non-selective beta-blockers reduce portal pressure and are the firstline treatment in patients with varices. Whether physical measurements of liver and spleen stiffness can reflect the response to beta-blockers remains unclear. The non-invasive tests can be used more appropriately when such longitudinal data become available.
Notes
Conflicts of Interest: Grace Wong has served as an advisory board member for Gilead and received paid lecture fees from AbbVie, Bristol-Myers Squibb, Echosens, Gilead and Janssen. Vincent Wong has served as an advisory board member for Gilead and Janssen and a consultant for AbbVie, Merck and NovoMedica, and received paid lecture fees from AbbVie, Echosens and Gilead.
Abbreviations
ALT
alanine aminotransferase
APRI
AST-to-platelet ratio index
AST
aspartate aminotransferase
CHB
chronic hepatitis B
CHC
chronic hepatitis C
EGD
esophagogastroduodenoscopy
ELF
enhanced liver fibrosis
HBV
hepatitis B virus
HCC
hepatocellular carcinoma
HCV
hepatitis C virus
HR
hazard ratio
HVPG
hepatic vein pressure gradient
LSM
liver stiffness measurement
NAFLD
non-alcoholic fatty liver disease