Highly effective peginterferon α-2a plus ribavirin combination therapy for chronic hepatitis C in hemophilia in Korea
Article information
Abstract
Background/Aims
Chronic hepatitis C (CHC) is a major comorbidity in patients with hemophilia. However, there are no published data on the efficacy of antiviral therapy in Korea. We assessed the safety and efficacy of combination therapy with peginterferon α-2a plus ribavirin for CHC in hemophilia.
Methods
Patients (n=115) were enrolled between March 2007 and December 2008. Seventy-seven patients were genotype 1 or 6, and 38 patients were genotype 2 or 3. We evaluated rapid virologic responses (RVRs), early virologic response (EVRs), end-of-treatment response (ETRs), sustained virologic response (SVRs), and relapses. Safety evaluations included adverse events and laboratory tests.
Results
Eleven patients were excluded from the study because they had been treated previously. Among the remaining 104 treatment-naïve patients, RVR was achieved in 64 (60.6%), ETR was achieved in 95 (91.3%), and SVR was achieved in 89 (85.6%). Relapse occurred in eight patients (8.9%). Common adverse events were hair loss (56.7%) and headache (51.0%). Common hematologic adverse events were neutropenia (22.1%), anemia (27.9%), and thrombocytopenia (3.8%). However, there were no serious adverse events such as bleeding. RVR was the only predictor of SVR in multivariate analysis.
Conclusions
Peginterferon α-2a plus ribavirin combination treatment produced a favorable response rate in CHC patients with hemophilia without serious adverse events.
INTRODUCTION
Hepatitis C virus (HCV) infection is a major cause of morbidity and mortality in patients with hemophilia. More than 90% of hemophiliacs who received non-virucidally treated factor concentrates prior to 1986 were infected with HCV. Because of their need for pooled factor concentrates, fresh frozen plasma or cryoprecipitate, which uses blood from multiple donors, hemophiliacs have the highest rate of infection of all those at high-risk of infection.12 Therefore, in previous studies the prevalence of HCV infection in hemophiliacs was 40-90%.3456
According to recent data, the prevalence of positive of anti-HCV is 19.5% in hemophiliacs in Korea.78 However, there are no known reports of long-term treatment outcome including the late relapse. So we assessed the efficacy and safety of peginterferon α-2a plus ribavirin therapy in hemophilic patients with chronic hepatitis C (CHC) in Korea.
MATERIALS AND METHODS
Patients
We enrolled one hundred and fifteen patients with hemophilia who were followed by the Korean hemophilia foundation hospital. The inclusion criteria were: 1) positive serum HCV RNA (>15 IU/mL) regardless of alanine transaminase (ALT) level during the six months before the study; and 2) aged eighteen years or older. Our exclusion criteria included the presence or history of any of the following: 1) decompensated liver disease, hepatocellular carcinoma or other malignant neoplastic disease; 2) liver transplantation; 3) severe cardiac or chronic pulmonary disease; 4) immunologically mediated disease; 5) retinopathy; or 6) poorly controlled psychiatric disease.
Liver biopsy was not required because the histological severity of hepatic disease was not a criteria for eligibility, and because this procedure is life threatening and very costly in hemophiliacs. Clinical evidence of cirrhosis was defined as signs of irregular margins of the liver on abdominal ultrasound or computed tomography (CT), endoscopic evidence of esophageal varices or hypertensive gastropathy, and/or enlarged portal vein or splenomegaly.
Some patients were naïve while others had previous treatment experience. However, we analyzed data of the treatment naïve patients because previous treatment experience could influence treatment outcomes.
A single unit of transfused factors was produced by extracting necessary factors from the blood of thousands of donors. Therefore, the duration of the infection was determined to start at the point of the first transfusion.
Study design
This study was designed as a single-center open-label one-arm observational study of peginterferon α-2a (Pegasys, Roche, Basel, Switzerland) and ribavirin (Copegus, Roche) combination therapy of CHC infection in hemophilia A. The duration on enrollment was 22 months, from March 2007 to December 2008. Eligible patients received 180 µg of peginterferon α-2a subcutaneously once a week in combination with 800-1,200 mg of oral ribavirin per day, according to their genotype and body weight. Patients with virus genotypes 1 or 6 with a weight below 75 kg were given 1,000 mg of oral ribavirin per day, while patients over 75 kg received 1,200 mg of oral ribavirin daily. Patients with virus genotypes 2 or 3 were given a constant ribavirin dose of 800 mg/day. The treatment duration for genotype 1 or 6 infections was 48 weeks, and 24 weeks for patients with genotypes 2 or 3.9
Assessment of efficacy
Hepatitis C virus genotyping was carried out using the restriction fragment mass polymorphism (RFMP) method. Viral load quantification was performed using the Cobas Amplicor HCV Monitor, v2.0 (Roche Diagnostics, Branchburg, NJ, USA) with a lower HCV RNA detection level of 15 IU/mL.
Serum HCV RNA was measured at weeks 0, 4, 12, 24, 28 and 48 in genotypes 2 and 3 infections. In genotypes 1 and 6, it was measured at weeks 0, 4, 12, 48, 52 and 72. Our primary end point was a sustained virologic response (SVR), defined as undetectable HCV RNA (<15 IU/mL) at 24 weeks after cessation of treatment. Our secondary efficacy end points were rapid virological response (RVR: undetectable HCV RNA at week 4 of treatment), complete early virological response (cEVR: undetectable HCV RNA at week 12 of treatment), partial EVR (pEVR: detectable HCV RNA level but a decrease of ≥2 log10 from the baseline level), end of treatment response (ETR: undetectable HCV RNA at the end of 24 or 48 weeks of treatment), null response (<2 log10 reduction of HCV RNA from baseline at 12 weeks of therapy), and relapse (reappearance of HCV RNA in serum after therapy is discontinued). Patients with an insufficient virological response at 12 weeks (a detectable HCV RNA level and a decrease of <2 log10 from baseline) in genotype 1 or without ETR in all genotypes were considered to have treatment failure, and were withdrawn from the treatment.9
Three types of HCV relapse were considered: (1) early, defined as an HCV RNA rebound occurring between the end of treatment and week 12; (2) late, defined as an HCV RNA rebound occurring between weeks 12 and 24; and (3) very late, defined as an HCV RNA rebound occurring beyond week 24.10
Assessment of safety
Safety was assessed by monthly laboratory tests and evaluation of adverse events during treatment and follow-up. Adverse events were graded by the investigators as mild, moderate, severe, or life-threatening, according to a modified World Health Organization grading system.
Non-life-threatening adverse events were managed by reduction of the dose of peginterferon or with a prescription of appropriate medications. The laboratory criteria for dose reduction of peginterferon were 500-750 neutrophils/mm3 or 30,000-50,000 platelets/mm3. Decline of neutrophil count below 500 cells/mm3, decline of platelet count below 30,000/mm3 and decline of hemoglobin below 7 g/dL (because of lack of tolerance) were considered as criteria for peginterferon discontinuation. The ribavirin dose was reduced when the hemoglobin level declined below 9 g/dL and was discontinued when it declined below 7.5 g/mL. Whenever patients had abnormal laboratory test results, in addition to dose adjustment, they were asked to recheck that laboratory test at 1 or 2 weeks intervals until it improved. Otherwise, the treatment was discontinued permanently.9
Statistical analysis
Summary statistics for virological outcomes are reported for subgroups of patients defined by their baseline characteristics. Analyses include data from all patients who received at least one dose of study medication. Logistic regression was used in univariate and multivariate analyses to predict factors related to SVR. P-values of less than 0.05 were deemed to be significant. Continuous variables were analyzed using the Mann-Whitney U test. In the tables, continuous variables are presented as median values (range, minimum value-maximum value), while qualitative and discrete variables are presented as absolute and relative frequencies as percentages. An intention-to-treat analysis was performed. All statistical analyses were conducted using SPSS version 14.0 for Windows (SPSS Inc., Chicago, IL, USA).
Ethics statement
The study protocol was approved by the institutional review board of Chung-Ang University College of Medicine (IRB No. 7-070-10-20). The data were analyzed anonymously.
RESULTS
Patient characteristics at baseline
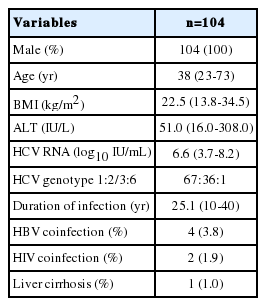
Between March 2007 and December 2008, a total of one hundred and fifteen patients with hemophilia were enrolled and treated. From the all studied patients, one hundred and four patients were treatment-naïve and received HCV antiviral therapy for the first time. Eleven patients who were receiving retreatment, five patients were relapsed and six patients were null responder to previous standard interferon and ribavirin combination therapy. All patients were hemophilia A males. The median age of the patients was thirty-eight years at the beginning of therapy. The median body mass index (BMI) was 22.5 (range, 13.8-34.5) kg/m2 and the median ALT level was 51.0 (16.0-308.0) IU/L. The median HCV RNA was 6.6 (3.7-8.2) log10 IU/mL. Among probable HCV genotypes, genotype 1 was the most frequent (64%) and the proportions of subtypes were 1a (8/67), 1b (54/67), 1c (3/67) and 1d (2/67). Thirty-five patients were genotype 2 -2a (23/35), 2b (3/35), 2a2b (1/35), 2a2c (8/35)- and there was one patient of each genotype 3and 6. In our study, the mean duration of infection was 25.1 years. Four patients had HBV-HCV co-infection and two patients had HIV-HCV co-infection. All HBV co-infections were negative for HBeAg and DNA undetectable and all HIV co-infections were in a stable state. Compensated cirrhosis was observed in one patient (Table 1).
Virological response rates and treatment regimen efficacy
Of the one hundred and four cases of HCV hemophilia, eighty-nine patients (85.6%) achieved SVR. The one patient who had genotype 1 did not achieve cEVR. Additionally, eight patients (7.7%) were prematurely withdrawn from treatment because of adverse events: five patients had a general weakness, one patient had jaundice (bilirubin increased to 18 mg/dL), one patient had an infection and one suffered from dizziness. Relapse occurred in eight patients (8.9%). Five were early relapsers and one was a late relapser (Fig. 1). Two patients relapsed during the very late period.

Clinical outcomes. CHC, chronic hepatitis C; RVR, rapid virologic response; EVR, early virologic response; ETR, end-of-treatment response; SVR, sustained virologic response; cEVR, complete early virological response.
Figure 2 shows the virological response by treatment duration for each genotype. As shown, the SVR rate of all patients was 85.6% (82.4% in genotypes 1/6 and 91.7% in genotypes 2/3). The EVR rate was similar between these two groups (P>0.05). However, we found that the RVR rate was significantly lower in genotype 1 and genotype 6.
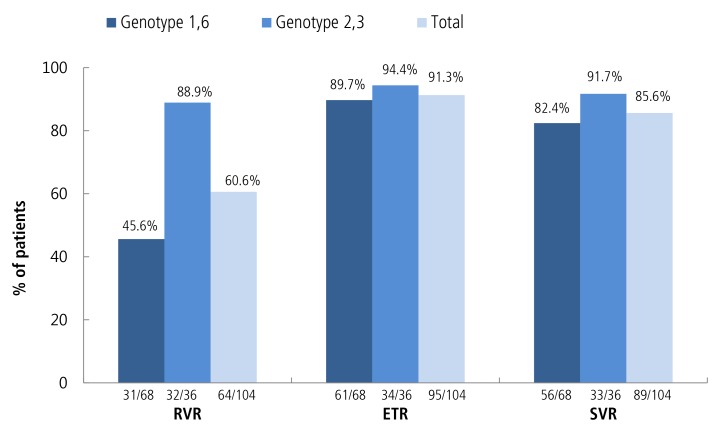
Virological response in treatment-naïve patients. Note: Response was determined based on the results of a treatment period using an intention-to-treat analysis. RVR, rapid virologic response; ETR, end-oftreatment response; SVR, sustained virologic response.
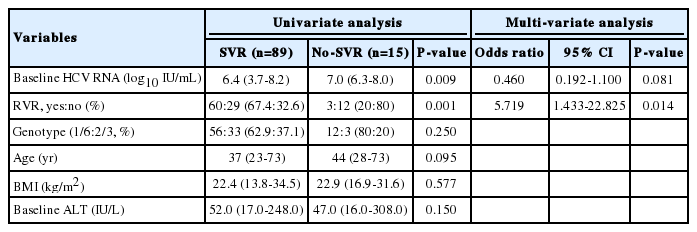
In univariate analysis, the baseline level of HCV RNA and achievement for RVR were predictive factors for SVR. However, in multivariate analysis, only achievement for RVR was identified as an independent predictor of SVR (Table 2). In addition, virological response in previous treatment-failure patients is shown in Table 3.
Drug doses and side effects
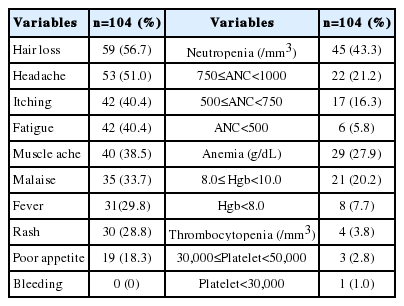
Adverse events are reported for the entire study population. The most common adverse events were hair loss, headache, itching, neutropenia and fatigue (Table 4). Most were mild, and only a few patients had severe hematologic events. The proportion of patients with severe neutropenia (a neutrophil count of <500/mm3) was 5.8%, anemia (Hgb <8 g/dL) 7.7% and thrombocytopenia (<30,000/mm3) 1%. Moreover, no patients experienced bleeding.
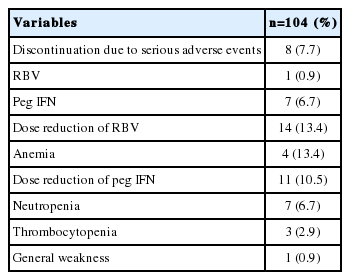
The proportion of patients who discontinued the treatment due to a serious adverse event was 7.7% (Table 5). The discontinuation of ribavirin occurred in one patient (0.9%) because of anemia and of peginterferon in seven patients (6.7%) because of general weakness, jaundice and infection. However, there were no patient who discontinued for cytopenia. The proportion of patients who underwent peginterferon-dose reduction was 10.5%: 6.7% for neutropenia, 2.9% for thrombocytopenia, and 0.9% for general weakness. The proportion of patients undergoing ribavirin-dose reduction was 13.4% (for anemia).
Virological response rates of coinfected patients
All of the HBV coinfected patients achieved SVR. All of them were hepatitis B carriers with a DNA undetectable state before and after the treatment. Only one patient showed a slight increase in the amount of virus after treatment, but it was still less than 10,000 copies/mL.
Of the two HIV coinfected patients, one patient achieved SVR and the other was withdrawn from treatment because of jaundice.
DISCUSSION
To our knowledge, this is the first study of long term clinical outcomes in patients with hemophilia and CHC treated with peginterferon plus ribavirin in Korea.
The standard treatment for CHC in 2008 was weekly subcutaneous peginterferon-α and daily oral ribavirin.911 The SVR rate was observed in 42-74% in hemophiliacs treated with the standard regimen (33-51% in genotype 1 patients, 71-86% in genotype 2 or 3 patients.13121314 Results for non-hemophiliacs were similar (42-52% in genotype 1, 76-84% in genotypes 2 or 3).1315
In our study, the SVR rate was 85.2% (81.8% in genotype 1, 92.1% in genotypes 2 or 3), higher than previous data. There were several reasons for the high SVR. First, there was good compliance. Because hemophilia is a rare disease in Korea, patients who registered in the Korean hemophilic foundation hospital have a good compliance. Most patients were able to follow up to the end of treatment and to the present. Second, almost all patients were treated using a standard dose. There were more young people than most studies of non-hemophilic patients, and most of them could tolerate the side effects. Because they did not have severe complications such as fever or bleeding, most patients were treated for a sufficient period of time to the standard regimen. Third, there were few patients with HIV co-infection. Genotype 1 and co-infection with HIV were strong predictors of a worse response to peginterferon therapy.11216 Furthermore, the treatment of patients with HBV co-infection was effective.
After the end of treatment and during long term follow up for about four years (to the present), it was possible to evaluate the rate and timing of relapse. Relapse occurred in eight patients (8.9%). Two relapsers occurred within 4 weeks of the end of treatment, three relapsers occurred within 12 weeks and one relapser occurred within 24 weeks. Two were very late relapsers, one at 40 weeks and the other at 72 weeks (both were genotype 1b). Few studies have mentioned relapsers treated with peginterferon plus ribavirin. In recent studies, the rate of relapse has been 0.9-22%.1718
The pEVR was not analyzed separately as a predictive factor of SVR because there was no statistical significance. pEVR occurred in four patients who had genotype 1b. Two of them did not achieve ETR, and the others relapsed.
A previous study showed that the favorable allele of IL-28 is more frequent in Asian patients compared to Caucasian patients, which may explain the superior response to interferon based therapies in Asian patients, including Koreans.51819 To determine if this is indeed the case, we plan to perform single nucleotide polymorphism genotyping of IL-28 with our samples in the future.
There are limitations of this study. The primary limitation is the small number of patients. Considering the fact that Korea has few hemophiliacs, it was not be easy to enroll in the study. The other major limitation is that this is a retrospective cohort study.
In conclusion, we showed that responses to antiviral therapy in patients with hemophilia appear to be superior to those in the general population. There were no serious adverse events related to hemophilia, such as bleeding.
Notes
Conflicts of Interest: The authors have no conflicts to disclose.
Abbreviations
ALT
alanine aminotransferase
CHC
chronic hepatitis C
ETR
end of treatment response
EVR
early virologic response
HBsAg
hepatitis B surface antigen
HBV
hepatitis B virus
HCV
hepatitis C virus
Hgb
hemoglobin
HIV
human immunodeficiency virus
RVR
rapid virologic response
SVR
sustained virologic response