Geographic differences in the epidemiological features of HCV infection in Korea
Article information
Abstract
Background/Aims
The prevalence of hepatitis C virus (HCV) infection in Korea exhibits significant geographic variation, with it being higher in Busan and Jeonam than in other areas. The reason for this intranational geographic difference was investigated in this study by conducting a comparative analysis of the risk factors related to HCV infection among three geographic areas: the capital (Seoul), Busan, and the province of Jeolla.
Methods
In total, 990 patients with chronic HCV infection were prospectively enrolled at 5 university hospitals located in Seoul (n=374), Busan (n=264), and Jeolla (n=352). A standardized questionnaire survey on the risk factors for HCV infection was administered to these three groups of patients, and a comparative analysis of the findings was performed.
Results
The analysis revealed significant regional differences in exposure to the risk factors of HCV infection. By comparison with patients in Seoul as a control group in the multivariate analysis, patients in Busan had significantly more experience of invasive medical procedures, acupuncture, cosmetic procedures, and multiple sex partners. In contrast, patients in Jeolla were significantly older, and they had a higher prevalence of hepatocellular carcinoma, a lower prevalence of multiple sex partners, and had experienced fewer invasive procedures.
Conclusions
There was a significant geographic difference in the exposure to potential risk factors of HCV infection between patients from the three studied regions. This may explain the regional variation of the prevalence of HCV infection in Korea, and should be taken into account when planning strategies for the prevention and management of HCV infection.
INTRODUCTION
Hepatitis C virus (HCV) is a major cause of chronic liver disease worldwide, and HCV-infected persons are at risk of developing liver cirrhosis and hepatocellular carcinoma. The global prevalence of HCV infection is estimated to be approximately 2.8%, affecting about 170 million individuals.1
There are significant global differences in HCV prevalence from less than 0.5% up to near 30%. In many developed countries where the prevalence of HCV is relatively low, HCV is mainly transmitted by intravenous drug use,2 while in developing countries with relatively high prevalence, unsafe parenteral procedures such as unsterilized syringe use are major risk factors.3,4 Therefore, the profiles of risk factors for HCV infection vary considerably in different countries or populations.
The prevalence of HCV infection in South Korea is reported to be 0.78-1.7%, which was increasing with age. The risk factors for HCV infection in South Korea includes transfusion before 1995, intravenous drug use, multiple sex partners, hemodialysis, needle stick injury, and tattooing.5,6 Interestingly, even in the South Korea, there was significant difference in HCV prevalence among the geographic regions, showing characteristically high prevalence in southern coastal area of Jeonman, Gyeonman and Busan.7,8 However, the cause of geographic difference of HCV prevalence in Korea is unclear. The different pattern of exposure to the above risk factors for HCV infection might explain the geographic difference of HCV prevalence in Korea. Moreover, elucidating the regional risk factors is the premise for the prevention of HCV infection.
To explain the reason of the intranational geographic difference of HCV prevalence, we performed comparative analysis of risk factors related to HCV infection among 3 geographic areas, including the Capital area, Busan, and Jeolla area.
MATERIALS AND METHODS
Patients
Patients who were aged >18 years and positive for anti-HCV antibody were prospectively enrolled from June 2010 to September 2014 at 5 university hospitals in South Korea. Two of the hospitals were located in a capital area (Seoul National University Bundang Hospital [SNBH] and Soonchunhyang University Bucheon Hospital [SUBH]), one in Busan (Inje University Busan Paik Hospital [BPH]), and the other two hospitals in Jeolla province (Chonbuk National University Hospital [CBUH] and Chonnam National University Hwasun Hospital [CNHH]). Although this HCV cohort was established since Janurary 2007,5 hospitals in Busan and Jeolla joined from June 2010, so that this study included a total of 1034 patients who enrolled from June 2010. Among the enrolled patients, patients with acute hepatitis C (n=26) or positive anti-HCV antibody but negative serum HCV RNA without antiviral treatment (n=18) were excluded. Therefore, a total of 990 patients (153 patients in SNBH, 221 in SUBH, 264 in BPH, 211 in CBUH, 141 in CNHH) with chronic HCV infection were included, the subjects were diagnostically classified as chronic hepatitis, cirrhosis and hepatocellular carcinoma (HCC). Cirrhosis of the liver was diagnosed according to the histological findings or radiological findings together with clinical features indicative of portal hypertension, such as thrombocytopenia (a platelet count <100,000/mm3), gastroesophageal varices, or ascites. HCC was diagnosed by histological examination or by typical radiographic findings consisting of arterial enhancement and venous wash-out on contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) of hepatic nodules.9 The subjects were prospectively followed at every 6 to 12 months.
Questionnaire survey on risk factors for HCV infection
A trained research coordinator at each hospital interviewed the subject patients using a standardized questionnaire, which included socio-demographics (age, gender, education, and occupation), health behaviors (smoking and drinking), and medical history including accompanying diseases (cancer, thyroid disease, psychiatric disease, diabetes, kidney disease, cerebrovascular disease, and cardiovascular disease). The patients answered the questions regarding their lifetime experience with theoretical risk factors for HCV infection, such as acupuncture, dental procedures, diagnostic endoscopy, surgery, other invasive medical procedures, tattooing, piercing, cosmetic procedures, needle stick injury, blood transfusion, hemodialysis, diagnosis of hemophilia, intravenous drug use, incarceration, familial history of HCV-related liver disease, living with HCV carriers, and number of sexual partners.
Completed questionnaires and clinical data were entered into the electronic case report form at the Korean HCV cohort study group homepage (http://www.hcvcohort.or.kr).
Statistical analysis
The subject patients were divided into 3 groups according to the location of the hospital; capital area (SNBH and SUBH), Busan area (BPH), and Jeolla area (CBUH, CNHH). Categorical variables were compared with the chi-square test, and continuous variables were compared with ANOVA test. To elucidate the risk factors specifically related to patients in Busan or Jeolla area compared to those in the capital area, univariate and multivariate multinomial analyses in which capital area was chosen as a reference category were performed. Multivariate multinomial logistic regression analyses were conducted to assess the difference of potential risk factors, comorbidity, age and sex which are significantly different in univariate logistic regression analyses. In these models, dependent variables such as age, gender, diagnostic category, intravenous drug use, needle stick injury, blood transfusion before 1995, surgery before HCV diagnosis, HBV coinfection, invasive medical procedures, living with HCV carriers, hemodialysis, number of sexual partners, dental procedures, diagnostic endoscopy, cosmetic procedure, tattooing, and piercing were included. Data were analyzed using SAS enterprise guide 5.1 software (SAS Institute, Cary, NC).
RESULTS
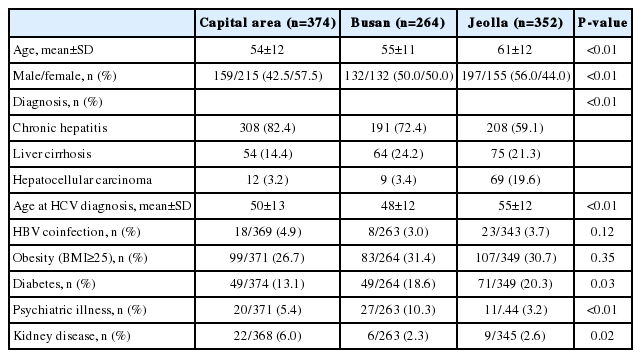
Comparison of demographic and clinical features among 3 groups of subjects based on the geographical location of the hospital
A total of 990 patients were included in this study. The mean age of the patients was 56.7 years, and 50.7% of patients were female. The diagnostic distributions of the patients were chronic hepatitis (n=707, 71.4%), cirrhosis (n=193. 19.5%) and HCC (n=90, 9.1%). The demographic and clinical features of the patients in 3 geographically different groups were compared in Table 1. The patients from Jeolla area (CBUH and CNHH) are older, more male proportion, and higher proportion of HCC, because CNHH is a regional cancer center. Diabetes is more prevalent in Jeolla, kidney disease in a capital area, and psychiatric illness, mainly depression in Busan (Table 1).
Comparison of potential risk factors for HCV infection among 3 different regions
The extent to exposure to potential risk factors of HCV infection is different among hospitals (Table 2). Experience of dental procedure and endoscopy, transfusion before 1995, surgery before HCV diagnosis, dialysis, birth from HCV-infected mother, invasive medical procedures, needle stick injury, acupuncture, intravenous drug use, multiple sexual partners, incarceration, tattooing, piercing, and cosmetic procedures were significantly different among 3 regions in chi-square analyses. Overall, patients in Busan were more exposed to the risk factors of HCV infection, whereas patients in Jeolla were less exposed to them. Among various invasive medical and cosmotic procedures, intraarticular injection and shaving in barber shops were significantly more frequent in Busan, respectively.

Comparison of potential risk factors for HCV infection among three different geographical regions of Korea
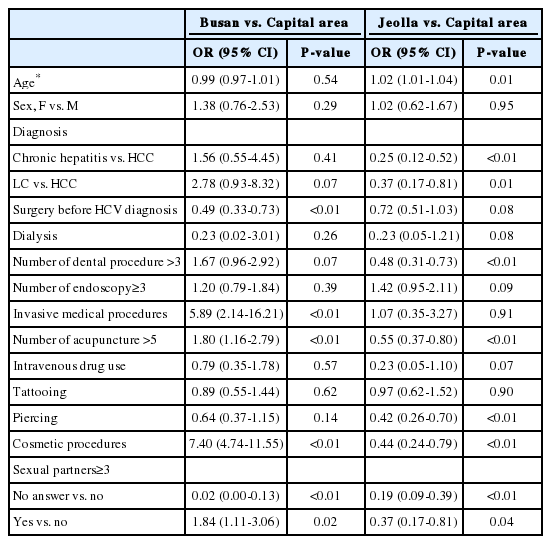
In univariate logistic regression, more patients in Busan had experience of dental procedures, endoscopy, dialysis, invasive medical procedures, acupuncture, piercing, cosmetic procedures, intravenous drug use, incarceration, and having multiple sex partners compared with those in capital area (Table 3), whereas less patients in Busan had experienced surgery before HCV diagnosis compared with those in capital area. In multinomial multivariate logistic regression analsysis, patients in Busan had significantly more experience of invasive medical procedures, acupunctures, cosmetic procedures and having multiple sex partners compared with those in capital area (Table 4).

Multinomial univariate analysis for the potential risk factors of HCV infection among three different geographical regions of Korea
Multinomial multivariate analysis for the potential risk factors of HCV infection among three different geographical regions of Korea
Patients in Jeolla had significantly less experience of surgery before HCV diagnosis, dialysis, dental procedures, acupuncture, tattooing, piercing, cosmetic procedures, intravenous drug use and incarceration, and having multiple sex partners compared with those in capital area in univariate analysis (Table 3). In multinomial multivariate analysis, significantly less patients in Homan had experienced dental procedures, acupuncture, piercing, and cosmetic procedures, and had multiple sex partners compared with those in capital area (Table 4).
DISCUSSION
The present study compared the risk factors for HCV infection among 3 groups of chronic HCV infected patients in 3 geographically different regions in Korea using questionnaire survey. As a result, the extent and content of exposure to the risk factors of HCV infection showed significant regional differences, suggesting a plausible explanation for the intranational regional variation of HCV prevalence in Korea.
The prevalence of HCV infection in Korea has a characteristic geographic variation. According to National Health Insurance database, Busan showed highest age-adjusted prevalence ratio (APR), 1.76, accompanied by Jeonam 1.48, Gyeongnam 1.31. Jeju 1.20, and Seoul 1.13 in order, while Jeonbuk has the lowest APR (0.42).8 The reason of the intranational geographic difference of HCV prevalence is still unclear. The different pattern of exposure to the risk factors of HCV might explain the geographic difference of HCV prevalence in Korea.
Potential risk factors for HCV transmission in Korea includes blood transfusion before HCV screening, intravenous drug use, hemodialysis, needle stick injury, tattooing, dental procedures, and multiple sexual partners.5,6
In this study, more patients in Busan had been exposed to risk factors for HCV infection including invasive medical procedure, acupuncture, cosmetic procedures, and multiple sex partners than those in capital area. Invasive procedure frequently performed in Busan was the intraarticular injection. Experience of cosmetic procedure was especially higher in male in Busan and was a shaving in barber shops. Shaving in barber shops was reported as a risk factor for HCV infection in other countries probably due to sharing unsterilized knives.10,11 There is no consensus on whether acupuncture is a risk factor for HCV transmission,6,12 but in this study, frequency as well as experience of acupuncture was higher in a region with high prevalence of HCV infection. Intravenous drug use seemed to be higher in Busan in univariate analysis, but not significantly different in multivariate analysis, probably because of interactions of closely related risk factors. Therefore, high prevalence of HCV infection in Busan might be explained by higher exposure to risk behaviors, suggesting that targets for preventive measures exist.
Interestingly, patients in Jeolla show significantly lower exposure to many potential risk factors, but were older compared with those in capital area. Old age might not come from CNHH's characteristic in which patients had more advanced disease, because patients in CBUH were also older. Patients in Jeolla had been less exposed to risk behaviors including dental procedure, acupuncture, piercing, cosmetic procedures, and multiple sexual partners than patients in capital area. Dental procedures were known to be a risk factor in Korea and foreign countries.6,13 Comparing two hospitals located in Jeolla province, patients in CNHH which is a local cancer center had more proportion of male, LC, HCC, and HBV coinfection and had been more exposed to risk factors for HCV infection including invasive medical procedures, dental procedures and multiple sex partners than those in CBUH where HCV prevalence was lowest in Korea (data not shown). It was difficult to explain the high prevalence of HCV infection in Jeolla with the exposure to risk factors. Considering the old age of the cohort, relatively high prevalence of HCV infection in this area might be remote cohort effect. As a reference group, two hospitals (SNBH and SUBH) located in the capital area did not show a significant difference of exposure to the risk factors (data not shown).
This study had several limitations. First, we did not identify current living address or birth place, and the region of hospitals might not be a place of residence. However, patients of hospitals located in non-capital area were not likely to reside in a different region, and approximately less than 10% of patients in the hospitals in capital area came from other region. Second, the subject patients were enrolled in university hospitals rather than primary care clinics, suggesting referral bias. Moreover, CNHH is a local cancer center, and has a high proportion of cirrhosis and HCC. This cohort might not exactly reflect the whole HCV patients. Third, some patients did not want to answer private questions such as those pertaining to sexual relationships, which resulted in missing data. We set an extra category for variables of which missing or unknown rates were over 10%. The response rates of questions about sexual behavior were significantly lower in subjects in capital area than those in Busan or Jeolla. Therefore, higher proportion of risky sexual behavior in Busan can be a reporting bias.
In conclusion, this study demonstrated the significant geographic difference of exposure to risk factors for HCV infection existed in Korea. The high prevalence of HCV infection in Busan may be related to various invasive procedures and high risk sexual behavior, therefore, active preventive measures to block the infection risk are urgently required. However, the high HCV prevalence in Jeolla province may be remote cohort effect, suggesting that active recognition of patients and treatment is a top priority. The difference of exposure to risk factors might explain a regional variation of HCV infection in Korea.
Acknowledgments
This study was supported by a grant for chronic infectious disease cohort study (Korea HCV Cohort study, 2014E5 100300) from the Korea Centers for Disease Control and Prevention. We are grateful to our research coordinators (Da Seul Lee, Da Woon Jeong, Ye Young Lee, Sun Mi Kim, Hyo Young Kang, Jeong-Wol Moon, Hui Bang, Young Soon Kim, Hyun Mi Kim, and Hyen Ji Yoon).
Notes
The authors have no conflicts to disclose.
Abbreviations
HCC
hepatocellular carcinoma
HBV
hepatitis B virus
HCV
hepatitis C virus
LC
liver cirrhosis