The comparison of esophageal variceal ligation plus propranolol versus propranolol alone for the primary prophylaxis of esophageal variceal bleeding
Article information
Abstract
Background/Aims
To investigate the efficacy and longterm outcome of esophageal variceal ligation (EVL) plus propranolol in comparison with propranolol alone for the primary prophylaxis of esophageal variceal bleeding.
Methods
A total of 504 patients were retrospectively enrolled in this study. 330 patients were in propranolol group (Gr1) and 174 patients were in EVL plus propranolol group (Gr2). The endpoints of this study were esophageal variceal bleeding and mortality. Association analyses were performed to evaluate bleeding and mortality between Gr1 and Gr2.
Results
EVL was more applied in patients with high risk, such as large-sized varices (F2 or F3) or positive red color signs. Total 38 patients had bleeds, 32 in Gr1 and 6 in Gr2. The cumulative probability of bleeding at 120 months was 13% in Gr1 versus 4% in Gr2 (P=0.04). The predictive factors of variceal bleeding were red color signs (OR 2.962, P=0.007) and the method of propranolol plus EVL (OR 0.160, P=0.000). 20 patients died in Gr1 and 12 in Gr2. Mortality rates are similar in the two groups compared, 6.7% in Gr1 and 6.9% in Gr2. The cumulative probability of mortality at 120 months was not significantly different in the two groups (7% in Gr1, 12% in Gr2, P=0.798). The prognostic factors for mortality were age over 50 (OR 5.496, P=0.002), Child-Pugh class B (OR 3.979, P=0.001), and Child-Pugh class C (OR 10.861, P=0.000).
Conclusions
EVL plus propranolol is more effective than propranolol alone in the prevention of the first variceal bleeding in patients with liver cirrhosis.
INTRODUCTION
Approximately half of patients with cirrhosis have esophageal varices, and one-third of all patients with varices will develop variceal bleeding, a major cause of morbidity and mortality in patients with cirrhosis.1,2 The determinants of variceal bleeding in cirrhotic patients are the size and appearance of the varices, and severity of hepatic dysfunction (Child-Pugh score).1 The mortality rate from first variceal bleeding remains very high (20-35%) despite aggressive management.3 There have been several medical, endoscopic, and surgical modalities to decrease the mortality rate from variceal bleeding. Preventive therapies of variceal bleeding due to portal hypertension are aimed at decreasing portal hypertension or treating the varices directly. Nonselective beta-blockers, surgical portal decompression, or transjugular intrahepatic portosystemic shunts can decrease portal hypertension, and esophageal variceal ligation (EVL) can treat the varices directly.4
It is recommended that nonselective beta-blockers are considered for patients with small varices which have never bled but have a high risk of bleeding (Child-Pugh class B, C or red color signs on endoscopy), and nonselective beta-blockers or EVL are considered for preventing the first bleeding occurrence in patients with large varices (F2 or F3).2,5 EVL has been shown to be safe and more effective than propranolol for the primary prevention of esophageal variceal bleeding.6,7 Theoretically, direct obliteration of varices by EVL in combination with reduction of portal pressure by propranolol would be more effective synergistically than either therapy applied alone. Actually, both EVL alone and combination of EVL and propranolol were effective in primary prophylaxis of bleeding from high-risk varices, although addition of propranolol did not decrease the probability of first bleeding or death in patients on EVL.8 But there is lack of sufficient data for the comparison of combination of EVL and propranolol versus propranolol alone for the primary prophylaxis of esophageal variceal bleeding. We compared the effect of EVL plus propranolol with that of propranolol alone in the prevention of first esophageal variceal bleeding and overall survival in cirrhotic patients with esophageal varices.
MATERIALS AND METHODS
Patients
A retrospective review of the medical records of cirrhotic patients with esophageal varices who had never bled and were treated with EVL plus propranolol or propranolol only for the primary prophylaxis of esophageal variceal bleeding in Samsung Medical Center from January 2003 to December 2012 was conducted. Inclusion criteria were the patients with liver cirrhosis with esophageal varices without history of hematemesis or melena. Liver cirrhosis was diagnosed either based on histology or on a combination of radiologic, laboratory, and clinical parameters. Patients were excluded if they presented with one or more of the following: the association with hepatocellular carcinoma or other malignancy; the association with stroke, chronic kidney disease, or other debilitating disease; concomitant gastric varices; history of liver transplantation or shunt surgery.
All patients underwent esophagogastroduodenoscopy. If varices were present, they were classified by using an endoscopic staging system: F1 - small, straight varices; F2 - enlarged, tortuous varices that occupy less than one-third of the lumen; F3 - large, coil-shaped varices that occupy more than one-third of the lumen.1 The etiology of liver cirrhosis was evaluated in all patients with viral markers for hepatitis B and C viruses, history of alcohol abuse. The severity of liver cirrhosis was classified by Child-Pugh score.
Propranolol administration
Propranolol was given orally every day and was started 10 mg twice a day. The dose of propranolol was adjusted for a reduction in the resting heart rate by 25%, to 55 beats per minute, or until the occurrence of side effects at each visit.
EVL
Variceal ligation was done by an endoscopy using a single-band or a multi-band ligation device as many bands as possible on the varices in the esophagus. EVL was done until obliteration of esophageal varices. Each session was performed on an in-hospital basis. All side effects and adverse events were recorded: especially post-procedure pain, bleeding from banding ulcers, and fever.
Endpoints
The primary end point was esophageal variceal bleeding. Secondary endpoint was death from any cause: esophageal variceal bleeding, causes related to the underlying liver disease, or unrelated causes. Variceal bleeding was defined as hematemesis and/or melena together with either endoscopic visualization of blood emitted from esophageal varices, or the presence of varices together with blood in the stomach and no other source of bleeding.
Statistical analysis
Quantitative results were expressed as mean ± SD unless otherwise stated and were analyzed using Student's 2-tailed t-test or Mann Whitney test as applicable. Qualitative results were analyzed by the χ2 test or Fisher's exact test. The probability of variceal bleeding and survival were analyzed by Gehan's generalized Wilcoxon method. Univariate analyses of baseline variables for bleeding and mortality were performed. Variables that were statistically significant on univariate analyses were then included in the multivariate analyses to identify independent prognostic factors: binary logistic regression model for predicting variceal bleeding; Cox regression model for predicting mortality. The level of statistical significance was taken at P<0.05. Statistical analyses was done using SPSS 20.0 software package (SPSS Inc., Chicago, IL).
RESULTS
Patients' characteristics
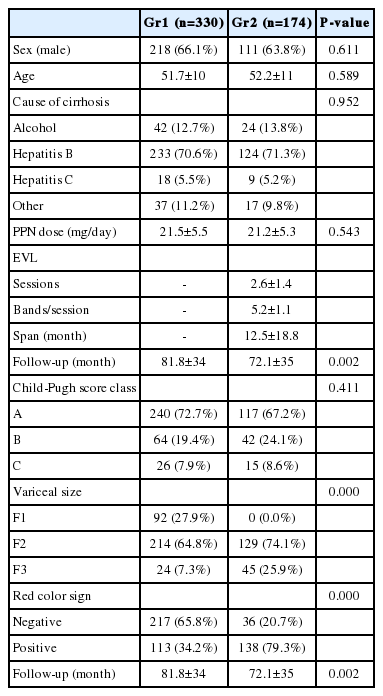
According to the criteria listed above, 504 consecutive patients were eligible for inclusion in this study. 330 patients were in propranolol only group (Gr1) and 174 patients were in EVL plus propranolol group (Gr2). Baseline characteristics are shown in Table 1. The two groups were comparable in respect to sex, age, cause of cirrhosis, propranolol dose, and Child-Pugh class. The mean follow-up in Gr1 was 81.8 ± 34 months and that in Gr2 was 72.1 ± 35 months (P=0.002). The mean daily propranolol used was 21.5 ± 5.5 mg in Gr1 and was 21.2 ± 5.3 mg in Gr2 (P=0.543). EVL was applied in patients with medium to large-sized varices such as F2 or F3. The mean number of EVL sessions in each patient was 2.6 ± 1.4 and the mean number of bands in each session was 5.2 ± 1.1 in Gr2.
Esophageal variceal bleeding
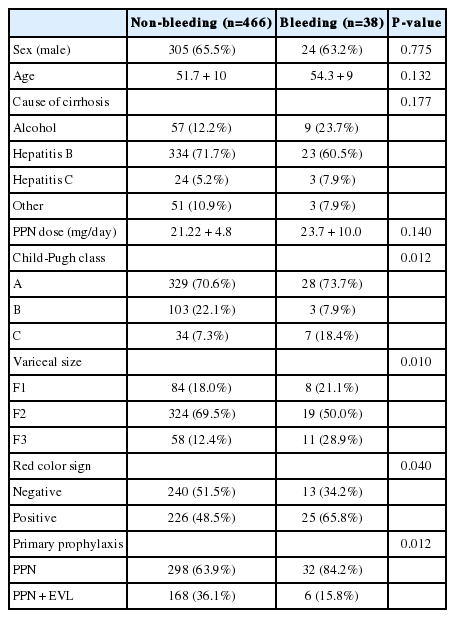
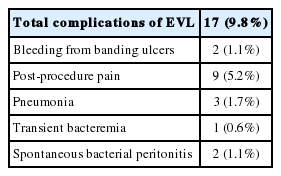
Variceal bleeding occurred in 32 (9.7%) patients in Gr1 and 6 (3.4%) patients in Gr2. Of total 38 bleedings, 28 (73.7%) occurred in Child's A, 3 (7.9%) in Child's B, and 7 (18.4%) in Child's C cirrhosis. The Gehan's generalized Wilcoxon method was used to estimate probability of bleeding. The patients with propranolol plus EVL had a lower probability of variceal bleeding. The cumulative probability of bleeding at 120 months was 13% in Gr1 versus 4% in Gr2 (P=0.04), indicating that the patients with EVL plus propranolol had a lower probability of variceal bleeding compared to propranolol alone (Fig. 1). Univariate analysis was performed to ascertain predictive factors of variceal bleeding by using baseline demographic, clinical, endoscopic characteristics, and the method of primary prophylaxis. Of all the factors analyzed, Child-Pugh class (P=0.012), variceal size (P=0.010), red color signs (P=0.040), and the method of primary prophylaxis (P=0.012) were associated with a high probability of variceal bleeding (Table 2). On multivariate analysis by binary logistic regression, red color signs (OR 2.962, P=0.007) and the prophylactic method of EVL plus propranolol (OR 0.160, P=0.000) were significantly related with esophageal variceal bleeding (Table 3).
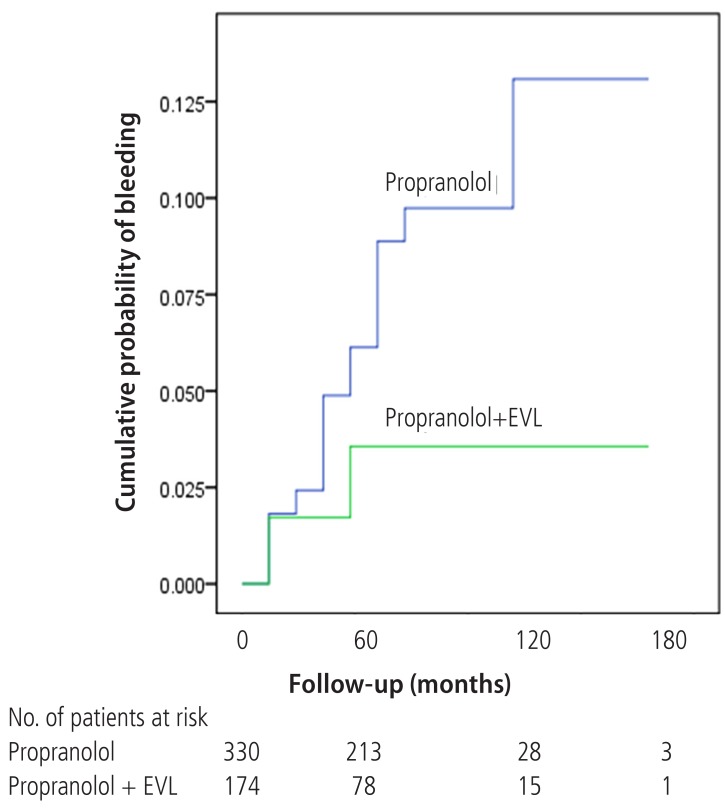
Wilcoxon plot showing the cumulative probability of variceal bleeding in patients with propranolol alone versus EVL plus propranolol (P=0.04).
Mortality
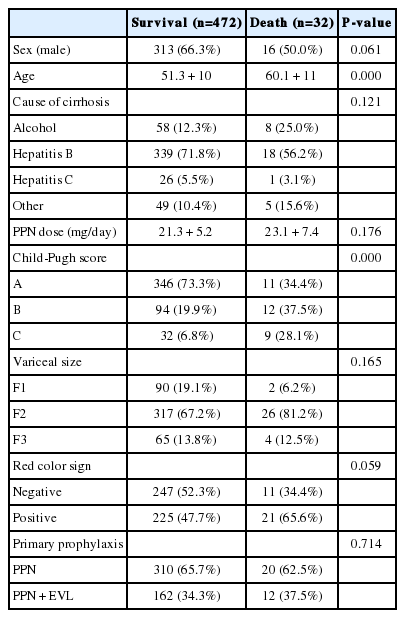
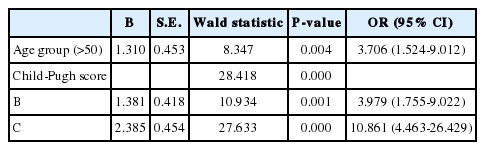
There were 20 deaths in Gr1 (6.1%) and 12 deaths in Gr2 (6.9%). Deaths from variceal bleeding occurred in 2 (0.6%) in Gr1 as compared to none in Gr2. Other causes of death were ascribed to end-stage liver disease (hepatic failure 4, hepatic encephalopathy 12, hepatorenal syndrome 3), infection (pneumonia 3, sepsis 3, spontaneous bacterial peritonitis 2), and lower gastrointestinal bleeding 3 (Table 4). The cumulative probability of mortality at 120 months was not significantly different in the two groups (7% in Gr1, 12% in Gr2, P=0.798). Overall survival plot for the two groups is shown in Figure 2. Univariate analysis was performed to ascertain predictive factors of deaths by using baseline demographic, clinical, endoscopic characteristics, and the method of primary prophylaxis. Of all the factors analyzed, age over 50 (P=0.000), and Child-Pugh class (P=0.000) were associated with a high probability of deaths (Table 5). On multivariate analysis by Cox regression, age over 50 (OR 5.496, P=0.002), Child-Pugh class B (OR 3.979, P=0.001), and Child-Pugh class C (OR 10.861, P=0.000) were identified as independent prognostic factors for mortality (Table 6).
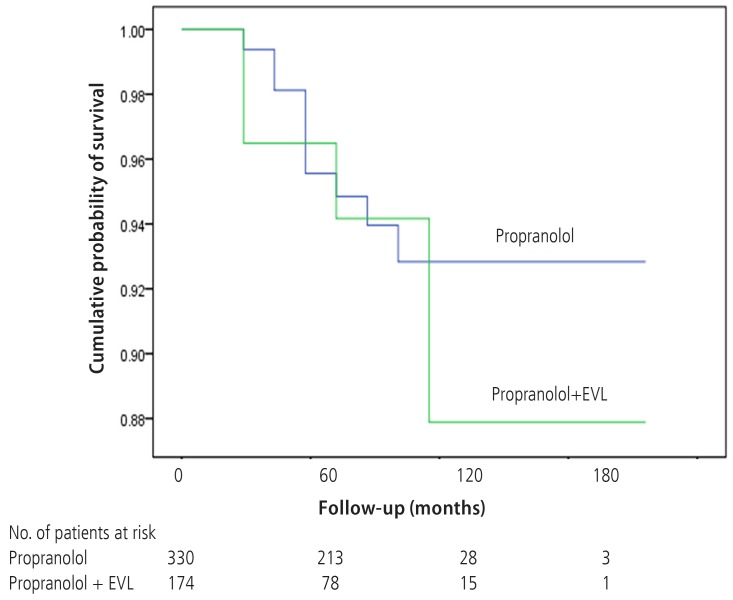
Wilcoxon plot showing the cumulative probability of survival in patients with propranolol alone versus EVL plus propranolol (P=0.789).
Adverse effects
Complications with EVL were defined as all events which followed on EVL in 2 weeks and could not be explained with other causes. They were as follows: bleeding from banding ulcers, post-procedure pain, pneumonia, transient bacteremia, and spontaneous bacterial peritonitis. But all of these resolved within 2 weeks of EVL, and none were fatal. No esophageal stricture or perforation was encountered (Table 7).
DISCUSSION
Nonselective beta-blockers or EVL have proved to be effective methods for primary prophylaxis of variceal bleeding. Nonselective beta-blockers can decrease portal pressure by reducing portal and azygos blood flow. Whereas, EVL diminishes variceal bleeding and the mortality rate by treating the varices directly.6,7,8,9 The aim of the present study was to investigate the efficacy and longterm outcome of EVL plus propranolol in comparison with propranolol alone for the primary prophylaxis of esophageal variceal bleeding.
Our results show that the incidence of first variceal bleeding in patients with liver cirrhosis is significantly reduced further by the addition of EVL to the propranolol administration. Variceal bleeding occurred in 32 (9.7%) patients in propranolol only group and 6 (3.4%) patients in EVL plus propranolol group. Accordingly, the cumulative probability of variceal bleeding was lower in EVL plus propranolol group than in propranolol only group (4% versus 13%, P=0.04).
In previously published meta-analyses, overall incidence of variceal bleeding was various in EVL group (0 to 25%) and in propranolol alone group (7 to 46%) and the mean daily propranolol dose was 30 to 113.5 mg.10,11,12,13,14,15 Prospective studies can control the daily propranolol dose tightly. But our clinical research was conducted retrospectively, so relatively low dose of propranolol was administered to the patients for fear that the patients should have serious side effects of the propranolol. So, the dose of propranolol might be not enough to control the portal pressure and therefore the bleeding control rate of Gr1 might be inferior to Gr2. However, our results on the efficacy of propranolol only for primary prophylaxis of variceal bleeding are comparable to those previously published studies in spite of relatively low dose of propranolol.
Clinical factors for predicting the risk of variceal bleeding in patients with liver cirrhosis are known such as location of varices,16 size of varices,1,17 appearance of varices,16,18,19 clinical features of the patient (age over 60 years, ascites, severe anemia, active alcoholism, etc)17,20 and variceal pressure.21,22 In our study, factors associated with increased risk of variceal bleeding were positive red color signs and the method of propranolol alone than EVL plus propranolol. Child's B, Child's C, and larger variceal size were also associated with variceal bleeding even if statistical significance was not achieved as defined.
Mortality rates are similar in the two groups compared, 6.7% in propranolol only group and 6.9% in EVL plus propranolol group. The cumulative probability of mortality at 120 months was different between the propranolol only group and the EVL plus propranolol group (7% versus 12%, P=0.789), although there was no statistical significance. The reason was that the mean follow-up in Gr1 was longer than that in Gr2 (81.8 ± 34 months versus 72.1 ± 35 months, P=0.002). Previous overall mortality was variously described in EVL group (0 to 51%) or propranolol alone group (0 to 43%).10,11,12,13,14,15 Of all the factors analyzed in our study, age over 50, poor liver function such as Child's B and Child's C were independent prognostic factors for survival. Several studies that compared EVL with nonselective beta-blockers have shown variable results. Certain of those studies showed a decreased risk of hemorrhage with EVL, while others did not.10,11,12,13,14,15 Overall, previous reports suggested that EVL was effective for primary prophylaxis and similar to nonselective beta-blockers with somewhat less hemorrhage but no changes in overall mortality. However, the reduced bleeding rates did not translate into reduced overall mortality for patients treated with EVL plus propranolol. Previous study suggested that EVL might not be a permanent therapy because varices could recur after initial eradication.6 In our study, the overall mortality rates between the two treatment groups were not significantly different but were influenced by age, and Child-Pugh class. Although infection-related deaths were more frequent in Gr2, complications with EVL resolved within 2 weeks of EVL and none were fatal. So it is hard to tell that infectious complications with EVL influenced on mortality.
Our study indicates that EVL plus propranolol appears to be superior to propranolol alone in the prevention of the first variceal bleeding in patients with liver cirrhosis. With EVL, varices can be obliterated possibly earlier, and therefore EVL offers advantage with that of propranolol therapy. Although EVL eradicates esophageal varices with fewer complications than endoscopic sclerotherapy,23 it must be kept in mind that EVL is inconvenience and has a potential for iatrogenic complications such as bleeding from banding ulcers, post-procedure pain, and infection, such as pneumonia, bacteremia, and spontaneous bacterial peritonitis.
Our study has limitations. First, this study was conducted as a retrospective study. So patients were not randomized to receive either EVL plus propranolol or propranolol and received EVL with different intervals between endoscopy. Also, EVL was performed on patients with F2 or F3 esophageal varices. Second, this study did not include specific information about recurrence of esophageal varices.
In conclusion, EVL plus propranolol is relatively safe and more effective than propranolol alone in preventing the first variceal bleeding in patients with liver cirrhosis. The combination of EVL and propranolol can be considered in preventing the first bleeding occurrence from esophageal varices in patients with high risk of bleeding (Child-Pugh class B, C or red color signs), being careful with complications of EVL.
Notes
The authors have no conflicts to disclose.
Abbreviations
EVL
esophageal variceal ligation