Cyclooxygenase-2 and vascular endothelial growth factor in chronic hepatitis, cirrhosis and hepatocellular carcinoma
Article information
Abstract
Background/Aims
Cyclooxygenase-2 (COX-2) and vascular endothelial growth factor (VEGF) are up-regulated in hepatocellular carcinoma (HCC). To investigate the levels of COX-2 and VEGF expression in chronic hepatitis (CH), cirrhosis, and HCC.
Methods
The immunohistochemical expressions of COX-2 and VEGF were evaluated in tissues from patients with CH (n=95), cirrhosis (n=38), low-grade HCC (LG-HCC; n=6), and high-grade HCC (HG-HCC; n=29).
Results
The COX-2 expression scores in CH, cirrhosis, LG-HCC, and HG-HCC were 3.3±1.9 (mean±SD), 4.2±1.7, 5.5±1.0, and 3.4±2.4, respectively (CH vs. cirrhosis, P=0.016; CH vs. LG-HCC, P=0.008; LG-HCC vs. HG-HCC, P=0.004), and the corresponding VEGF expression scores were 0.9±0.8, 1.5±0.7, 1.8±0.9, and 1.6±1.1 (CH vs. cirrhosis, P<0.001; CH vs. LG-HCC, P=0.011; LG-HCC vs. HG-HCC, P=0.075). Both factors were correlated with the fibrosis stage in CH and cirrhosis (COX-2: r=0.427, P<0.001; VEGF: r=0.491, P<0.001). There was a significant correlation between COX-2 and VEGF in all of the tissue samples (r=0.648, P<0.001), and between high COX-2 and VEGF expression scores and survival (COX-2: P=0.001; VEGF: P<0.001).
Conclusions
The expressions of both COX-2 and VEGF are significantly higher in cirrhosis and LG-HCC than in CH. High COX-2 and high VEGF expressions are associated with a high survival rate.
INTRODUCTION
Cyclooxygenase-2 (COX-2) is not expressed constitutively, but is induced rapidly by both inflammatory and mitogenic stimuli, resulting in increased prostaglandin (PG) synthesis in inflamed and neoplastic tissues.1 COX-2 overexpression has been reported in various types of cancers, based on these pathogeneses. Several studies have also suggested a relationship between the progression of chronic liver disease and hepatocarcinogenesis. Angiogenesis is essential for carcinogenesis and is induced directly by vascular endothelial growth factor (VEGF), leading to tumor growth and metastasis.2 Ischemic changes stimulate angiogenesis in the cirrhotic liver and in hepatocellular carcinoma (HCC). Hypervascular tumors are a key to the hypothesis that VEGF is overexpressed relative to the stage of liver disease and hepatocarcinogensis. For the early detection and prevention of the progression of liver disease, an exact understanding of the relationship between COX-2 and VEGF expression in liver disease is essential. However, their roles in the progression of chronic liver disease and hepatocarcinogenesis are not clearly understood. Here, we assessed the degree of COX-2 and VEGF expression in chronic hepatitis, liver cirrhosis, and HCC, to investigate the association between COX-2 and VEGF in the progression of chronic liver diseases.
MATERIALS AND METHODS
Materials
US-guided fine-needle liver biopsies were obtained from 168 patients between October 2003 and October 2009. They consisted of 95 cases of chronic hepatitis (56 hepatitis B virus, HBV; 39 hepatitis C virus, HCV), 38 cases of liver cirrhosis (22 HBV, 5 HCV, 6 alcohol, and 5 others), and 35 cases of HCC (26 HBV, 2 HCV, 1 alcohol, and 6 others). Pathological diagnosis was confirmed by an expert hepatopathologist. In chronic hepatitis and cirrhosis, the presence of inflammation, fibrosis and cirrhotic regenerative nodules was confirmed, respectively. The histological classification of HCCs was as follows: 6 low grade (well-differentiated type) and 29 high grade (moderately differentiated, poorly differentiated, and undifferentiated).
The clinical characteristics of the types of liver disease are summarized in Table 1. Informed consent was obtained from each patient or family member. This study was approved by the institutional review board of Soonchunhyang University, Seoul Hospital, Seoul, Korea.
COX-2 immunohistochemical staining
Biopsy samples were fixed in 10% neutral formalin and embedded in paraffin. Tissue sections were deparaffinized in xylene for at least 20 min and hydrated sequentially in 100%, 95%, 90%, and 80% ethanol solutions. After rinsing with water for 5 min, the sections were pretreated with ethylenediaminetetraacetic acid (EDTA) buffer (pH 6.0) for 12 min using a microwave antigen retrieval procedure. After rinsing, endogenous peroxidase activity was blocked by treatment with 3% hydrogen peroxide for 20 min. A primary mouse monoclonal antibody against COX-2 (1:100; Cayman Chemical, Ann Arbor, MI, USA) was applied to the sections for 1 h at room temperature. After rinsing with phosphate-buffered saline (PBS), the slides were incubated with a secondary antibody for 10 min at room temperature and rinsed with PBS. The sections were incubated in tertiary anti-horseradish peroxidase (HRP) conjugate for 10 min, rinsed in PBS, and incubated with diaminobenzidine (DAB) for a further 10 min. After counterstaining with Meyer's hematoxylin, the slides were mounted with Crystal Mount® (Biomeda, Foster City, CA, USA). Colon cancer tissue was used as a positive control. The colon cancer tissue for the negative control slide was processed in the same way, except that PBS was used instead of the primary antibody.
VEGF immunohistochemical staining
Tissue sections were deparaffinized in xylene for more than 20 min and sequentially hydrated in 100%, 95%, 90%, and 80% ethanol solutions. After rinsing with water for 5 min, the sections were pretreated with EDTA buffer (pH 8.0) for 20 min using a microwave antigen retrieval procedure. After rinsing, the endogenous peroxidase activity was blocked by treatment with 3% H2O2 for 20 min. The primary mouse monoclonal antibody against VEGF (Santa Cruz, U.S.A.), diluted to 1:300 strength, was applied to the sections for 1 h at room temperature. After rinsing with PBS, the slides were incubated with secondary antibody for 10 min at room temperature and rinsed with PBS. The sections were incubated in tertiary anti-HRP conjugate for 10 min, rinsed in PBS, and incubated with DAB for 10 min. After counterstaining with Meyer's hematoxylin, the slides were mounted with Crystal mount® (Biomeda, CA, USA).
Fibrosis and immunohistochemical staining scores
All stained biopsy samples were reviewed and interpreted by an expert hepatopathologist. Histological fibrosis staging was assessed using Ishak's system.3 The COX-2 and VEGF immunohistochemical staining scores were assessed using the scoring system of Qiu et al4 based on the sum of two parameters: intensity and distribution. As per the scoring system of Qiu et al,4 the intensity of COX-2 staining in the tissue was scored on a scale of 0-3 (0=negative staining, 1=weakly positive staining, 2=moderately positive staining, and 3=strongly positive staining; Fig. 1). Trace staining intensity and less than 5% positive cells were interpreted as negative; only definite dark brown staining was counted. The percentage of positive cells in each specimen was estimated and scored on a scale of 0-4 (0=negative, 1=positive staining in ≤25% of cells counted, 2=positive in >25% and ≤50%, 3=positive in >50% and ≤75%, and 4=positive in >75%). For each section, the sum of these two parameters was determined; the combined scores for low COX-2 expression were between 0 and 5, while high COX-2 expression scores were 6 or 7. For VEGF immunohistochemical staining, dark brown granular staining in the cytoplasm of hepatocytes was considered a positive finding. Staining intensity was graded as negative and trace (grade 0), weak (grade 1), medium (grade 2), and strong (grade 3), following Chen et al (Fig. 2).5 Low VEGF expression scores were 0 or 1, and high VEGF expression scores were 2 or 3.
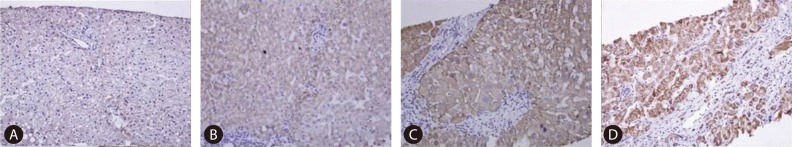
Immunohistochemical findings of COX-2 expression. (A) 0 (negative; mag. ×100), (B) 1 (weak; mag. ×200), (C) 2 (moderate; mag. ×200), and (D) 3 (strong; mag. ×200). COX-2, cyclooxygenase-2.
Statistical analysis
Student's t-tests were used to compare the COX-2 and VEGF expression scores between each pair of diseases. The chi-square test was used to compare the percentage of high scores for COX-2 (COX-2 ≥6) and VEGF (VEGF ≥2) expression between each pair of diseases. Pearson's correlation coefficient was used to examine the correlation between COX-2 and VEGF and the correlation between both COX-2 and VEGF and the fibrosis stage. Data are presented as the mean±standard deviation (SD). Statistical significance was accepted when P<0.05.
RESULTS
COX-2 expression
The mean COX-2 expression score in chronic hepatitis, cirrhosis, HCC was 3.3±1.9, 4.2±1.7, and 3.8±2.3, respectively. Subgrouping with HCC into low-grade HCC, and high-grade HCC, the mean COX-2 expression score in chronic hepatitis, cirrhosis, low-grade HCC, and high-grade HCC was 3.3±1.9, 4.2±1.7, 5.5±1.0, and 3.4±2.4, respectively (Fig. 3). The COX-2 expression scores in cirrhosis and low-grade HCC were significantly higher than in chronic hepatitis (chronic hepatitis vs. cirrhosis, P=0.016; chronic hepatitis vs. low-grade HCC, P=0.008). The COX-2 expression in low-grade HCC tended to be higher than in cirrhosis, but not significantly (P=0.084). Expression scores in high-grade HCC were significantly lower than in low-grade HCC (P=0.004). A significant difference was not observed between the high-grade HCC group and the chronic hepatitis group (P=0.782).
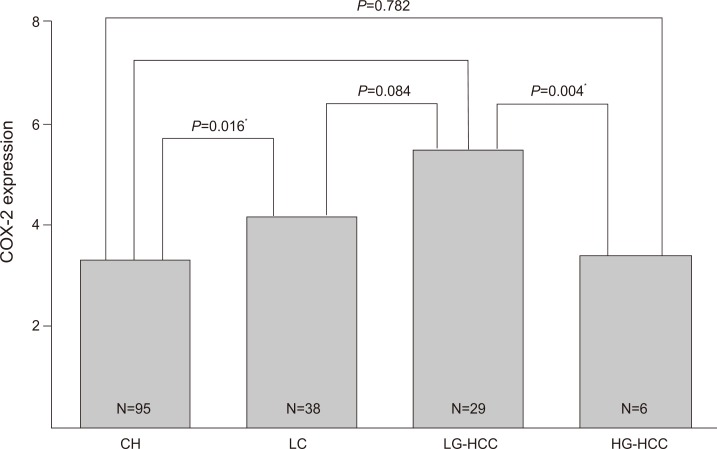
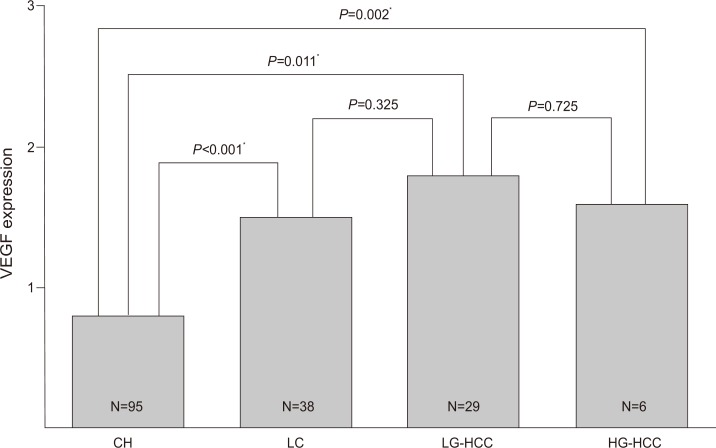
Immunohistochemical expression of COX-2 among the four liver-disease groups. CH, chronic hepatitis; LC, liver cirrhosis; HCC, hepatocellular carcinoma; LG, low-grade HCC; HG, high-grade HCC. *P<0.05.
The mean high expression COX-2 score (6-7) in all of the tissue samples, without grouping was 6.3±0.5, mean low expression COX-2 score (0-5) in all of the tissue samples was 3.1±1.8. The percentage of high COX-2 expression scores in chronic hepatitis, cirrhosis, and HCC was 9.4, 23.6, and 25.7%, respectively. The high COX-2 expression scores in cirrhosis and HCC were significantly higher than in chronic hepatitis (chronic hepatitis vs. cirrhosis, P=0.03; chronic hepatitis vs. HCC, P=0.017).
VEGF expression
The mean COX-2 expression score in chronic hepatitis, cirrhosis, HCC was 0.8±0.8, 1.5±0.7, 1.7±1.1, respectively. With subgrouping with HCC into low-grade, and high-grade, the mean VEGF expression score in chronic hepatitis, cirrhosis, low- and high-grade HCC was 0.8±0.8, 1.5±0.7, 1.8±0.9, and 1.6±1.1, respectively (Fig. 4). The VEGF expression scores in cirrhosis (P<0.001), and high-grade HCC (P=0.011) were significantly higher than in chronic hepatitis. The mean high expression VEGF score (2-3) in all of the tissue samples, without grouping was 2.2±0.4, mean low (0-1) expression VEGF score in all of the tissue samples was 0.49±0.5. The percentage of high VEGF expression scores in chronic hepatitis, cirrhosis, and HCC were 26.3, 52.6, and 65.7%, respectively. The high VEGF expression scores in cirrhosis and HCC were significantly higher than in chronic hepatitis (chronic hepatitis vs. cirrhosis, P=0.004; chronic hepatitis vs. HCC, P<0.001).
COX-2 and VEGF expression according to fibrosis stage
The mean score for fibrosis expression in both the chronic hepatitis and the cirrhosis group, without grouping, was 3.62±1.8. In same group, the mean expression score for COX-2 was 3.58±1.9, that for VEGF was 1.05±0.8. The mean expressions of COX-2 and VEGF according to the fibrosis grade score were as follows: fibrosis grade 0, COX-2=0±0, VEGF=0±0; grade 1, COX-2=1.5±1.5, VEGF=0.33±0.8; grade 2, COX-2=3.08±1.7, VEGF=0.62±0.8; grade 3, COX-2=3.91±1.8, VEGF=1.13±0.8; grade 4: COX-2=4.67±1.2, VEGF=1.67±0.8; grade 5, COX-2=4.31±1.4, VEGF=1.56±0.8; grade 6: COX-2=4.47±1.8, VEGF=1.59±0.9. There was a significant association between both COX-2 and VEGF expression levels and the stage of fibrosis in chronic hepatitis and cirrhosis (COX-2: r=0.430, P<0.001, VEGF: r=0.476, P<0.001).
In chronic hepatitis group, the mean score for fibrosis expression was 2.75±1.3, and the mean expression score for COX-2 was 3.33±1.9, that for VEGF was 0.87±0.9. The mean expressions of COX-2 and VEGF according to the fibrosis grade score were as follows: fibrosis grade 0, COX-2=0±0, VEGF=0±0; grade 1, COX-2=1.35±1.5, VEGF=0.18±0.5; grade 2, COX-2=3.04±1.8, VEGF=0.60±0.8; grade 3, COX-2=4.03±1.7, VEGF=1.13±0.8; grade 4: COX-2=4.50±1.2, VEGF=1.63±0.9; grade 5, COX-2=4.17±1.6, VEGF=1.33±0.8; grade 6: COX-2=5.00±1.4, VEGF=1.00±0.0.
In cirrhosis group, the mean score for fibrosis expression was 5.86±0.42, and the mean expression score for COX-2 was 4.21±1.73, that for VEGF was 1.5±0.7. The mean expressions of COX-2 and VEGF according to the fibrosis grade score were as follows: fibrosis grade 4, COX-2=6±0, VEGF=2±0; grade 5, COX-2=4.67±1.5, VEGF=2.33±0.6; grade 6, COX-2=4.18±1.7, VEGF=1.42±0.7.
Correlation between COX-2 and VEGF expression
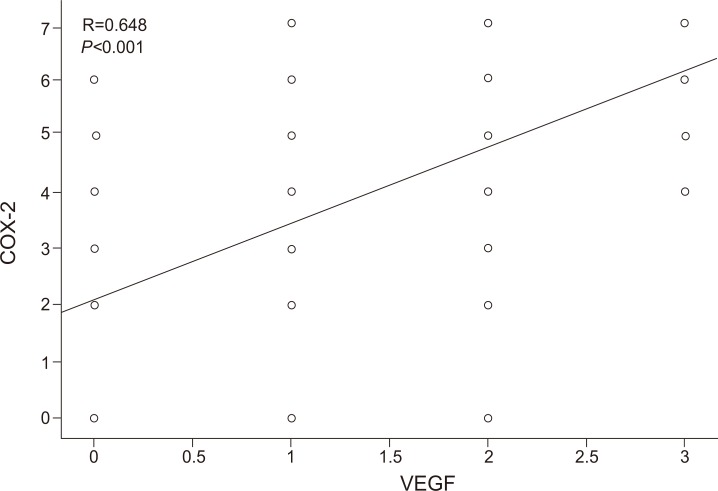
There was a positive correlation between COX-2 and VEGF expression levels in chronic hepatitis group (r=0.665, P<0.001), cirrhosis group (r=0.668, P<0.001), HCC group (r=0.603, P<0.001), and all of the tissue samples, without grouping (r=0.648, P<0.001) (Fig. 5).
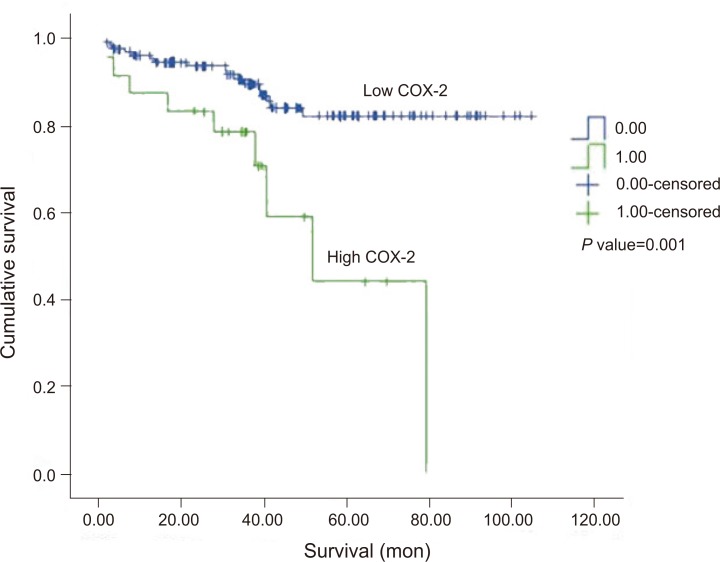
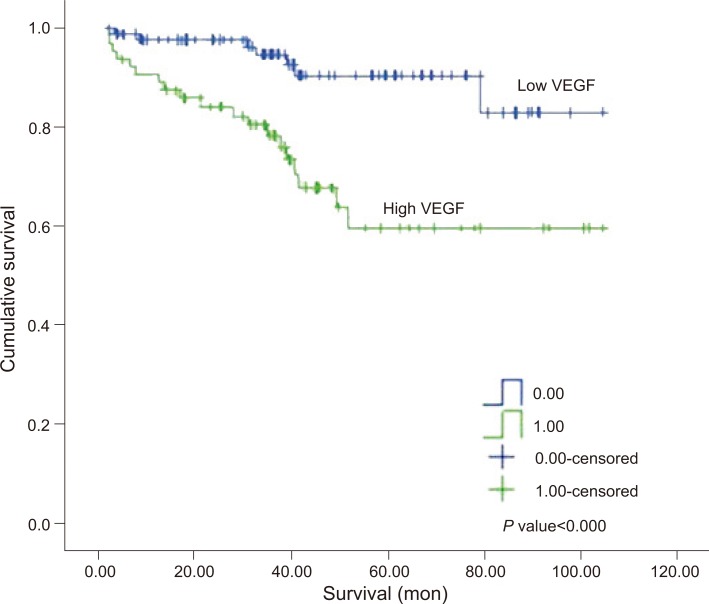
Survival rate according to COX-2 and VEGF expression
The rate of survival was lower in high COX-2 group and high VEGF group than in low COX-2 group and low VEGF group, respectively. Kaplan-Meier survival curve showed that there was a definite difference in overall survival between two groups according to COX-2 and VEGF level (the specific value are shown in Figs. 6, 7). The median follow-up time was 37.2 months (range, 1.7 to 104.8 months), and 5 patients were lost to follow-up.
DISCUSSION
We examined COX-2 and VEGF expression using immunohistochemical staining in liver tissues of patients with chronic hepatitis, cirrhosis, and HCC.
For COX-2, the cirrhosis and the low-grade HCC group showed significantly higher COX-2 expression compared to the chronic hepatitis group. The expression scores in high-grade HCC were significantly lower compared to the low-grade HCC. Chronic inflammation and related fibrosis have long been recognized as risk factors for the development of cancer, and COX-2 is thought to mediate the subsequent liver damage.6 Accordingly, the effects of COX-2 overexpression on the progression and hepatocarcinogenesis of chronic liver disease have been studied.4,7 Koga et al demonstrated that COX-2 was overexpressed in chronic hepatitis, liver cirrhosis, and early well-differentiated HCC and suggested that COX-2 plays a role in the early stages of hepatocarcinogenesis, which as a consequence may be related to HCC differentiation.8 Recent studies have revealed COX-2 overexpression in hepatitis, cirrhosis, and HCC;9,10 however, whether there is correlation between the progression of chronic liver disease and COX-2 remains unclear. In experimental study, COX-2 expression is upregulated in serum-starved hepatic stellate cell (HSC) forming extracellular matrix deposition, such as collagen. Mallat et al suggested HSC migration and proliferation in chronic liver disease stimulated by platelet-derived growth factor (PDGF) are associated with COX-2 induction and increased prostaglandin E2 production.11 In the present study, we demonstrated a definite difference between chronic hepatitis compared to each cirrhosis group and low-grade HCC, confirming previous studies. However, the differences between chronic hepatitis and high-grade HCC were not statistically significant, as reported by Koga et al.8 Koga presented a hypothesis to explain why COX-2 is overexpressed in low-grade HCC but not in high-grade HCC, suggesting that COX-2 plays a role in hepatocyte differentiation and the initiation of early hepatocarcinogenesis via the activation of the TGF-alpha/EGFR system; we believe our results support this hypothesis.
For VEGF, the cirrhosis group and each of the HCC grade groups expressed significantly more VEGF than the chronic hepatitis group. There was also a significant difference between the chronic hepatitis and the high-grade HCC group, a trend not observed in COX-2 expression. VEGF is a specific stimulator of endothelial cell proliferation in many human cancers, and is the most potent tumor angiogenic factor. Shi et al summarized VEGF overexpression and its hypothesized role in liver disease (including acute hepatitis, chronic hepatitis, liver cirrhosis, and primary and secondary HCC) as well as its inflammatory role in hepatitis, its fibrotic role in cirrhosis, and its neovascular role in carcinogenesis.12 Subsequently, Jerzy et al suggested that VEGF serum and receptor levels reflect the degree of impairment of hepatic function in liver cirrhosis.13 Further studies have examined the role of VEGF in the progression of chronic liver disease and carcinogenesis.14-16 Continuous VEGF expression throughout the course of HCC, proven by our results, supports the findings of these previous studies. With respect to molecular mechanism, Uematsu suggested hypoxia-induced angiogenesis through transcriptional activation of VEGF during hepatocarcinogenesis and cirrhotic change.17 But exact mechanism remained unclear so far.
In the present study, both COX-2 and VEGF were correlated with the fibrosis stage or Ishak's score, in chronic hepatitis and cirrhosis. Recently, there have been conflicting reports concerning both COX-2 and VEGF in fibrosis.18 The exact mechanism of action is unclear and additional studies investigating the roles of COX-2 and VEGF in the progression of fibrosis are required.
We also observed an overall positive correlation between COX-2 and VEGF expression levels in chronic hepatitis, cirrhosis, HCC group and all of the tissue samples. In addition to inflammation and related fibrosis, a correlation between COX-2 and angiogenesis, which is an essential process in tumor progression mediated by VEGF, has been reported in many studies of various tumors.19 COX-2 and VEGF overexpression has also been demonstrated in HCC, which is a highly malignant tumor characterized by active neovascularization.20 Nevertheless, this correlation does not explain the exact mechanisms involved. Toomey et al reported that the correlation may not be causative, but that COX-2 and VEGF share an uncertain common regulatory pathway.19 Further studies have suggested that parts of the mechanism include the COX-2/VEGF-dependent pathway.21 In experimental study using human hepatocelluar carcinoma inoculated nude mice, expression levels of both genes and proteins of COX-2 and VEGF by reverse transcription polymerase chain reaction and western blot were decreased in interferon-alpha-2b treatment group than in control group. With this study, we can presume COX-2 correlate with VEGF, in directly.22 The fact that low COX-2 and VEGF expression group survived longer than high COX-2 and VEGF expression group was demonstrated in our result. This is meaningful because our result suggested expression degree of COX-2 and VEGF as prognostic factor related with chronic liver disease progression.
The present study is associated with our previous study on the overexpression of COX-2 in the progression of hepatic fibrosis.23 Here we have also demonstrated that both COX-2 and VEGF are overexpressed in the progression of chronic liver disease, and that there is a correlation between them and, possible prognostic role of them. To the best of our knowledge, this is the first study to show positive correlations between COX-2 and VEGF in tissues of chronic hepatitis, cirrhosis, and HCC using immunohistochemical staining.
A limitation of this study is that a cross-sectional study cannot demonstrate a causal relationship for the high COX-2 and VEGF expression in advanced fibrosis, cirrhosis, and hepatocellular carcinoma. Secondly, size of low-grade HCC group is small compared with other groups. We think this fact may have an effect on our statistical result. Finally, about difference of survival rate, we did not consider other confounding factors can affect survival rate except COX-2 and VEGF expression rate.
In conclusion, COX-2 and VEGF expression were significantly higher in cirrhosis and low-grade HCC than in chronic hepatitis, and were correlated with the stage of fibrosis. There was a significant correlation between COX-2 and VEGF expression. In addition, there was a significant correlation between high COX-2 and VEGF expression score and survival. Thus, COX-2 and VEGF may play roles in the progression of liver disease and hepatocarcinogenesis via an unresolved mechanism. Further study is needed to clarify these relationships.
Notes
The authors have no conflicts to disclose.
Abbreviations
CH
chronic hepatitis
COX-2
cyclooxygenase-2
DAB
diaminobenzidine
EDTA
ethylenediaminetetraacetic acid
HCC
hepatocellular carcinoma
HG
high-grade hepatocellular carcinoma
HRP
anti-horseradish peroxidase
LG-HCC
low-grade hepatocellular carcinoma
PBS
phosphate-buffered saline
PDGF
platelet-derived growth factoer
PG
prostaglandin
SD
standard deviation