Solitary necrotic nodule of the liver
Article information
INTRODUCTION
Hepatic solitary necrotic nodule (SNN) is an unusual, benign mass forming lesion, first described by Shepherd and Lee in 1983.1 Since then, some possible pathogenetic hypotheses have been suggested. However, its exact etiology is still not established and disease entity of hepatic SNN remains uncertain. Although most SNNs show benign clinical course, it is difficult to distinguish from other lesions, including hepatic tumors and cancer metastasis by clinically and radiologically. In this issue, we present a case of SNN in a 48-year-old woman and discuss the histologic findings.
CASE SUMMARY
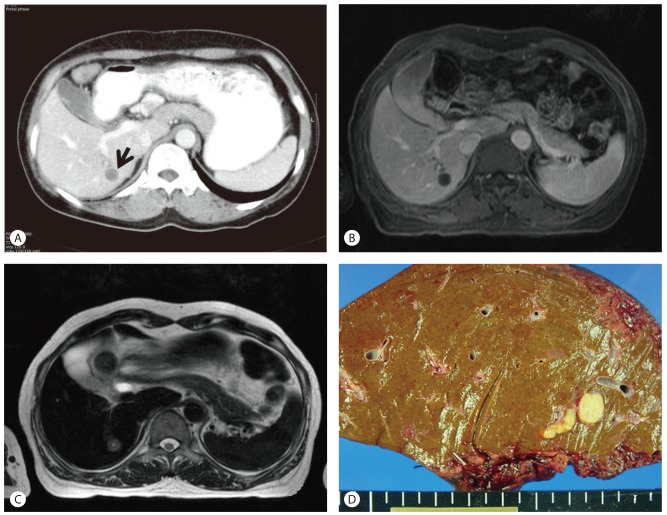
A 48-year-old woman visited our hospital for abdominal pain of one year's duration. The patient had a history of twice previous cesarean section delivery 25 years ago. Physical examination was unremarkable. The serum level for aspartate aminotransferase was 21 IU/L (normal range, 12-33 IU/L) and alanine aminotransferase was 12 IU/L (normal range, 5-35 IU/L), and other liver function tests were within normal limits. Serum tumor markers, including alpha-fetoprotein, carcinoembryonic antigen and carbohydrate antigen 19-9 were within normal limits. Serologic tests for hepatitis B and hepatitis C virus were negative. The complete blood count revealed an anemia, but leukocytosis and eosinophilia were not noted. Abdominal computed tomography (CT) scan demonstrated a 1.5×1.5 cm sized hepatic nodule located in segment VI of the right lobe, with differential diagnosis of cholangiocarcinoma, hepatocellular carcinoma, and metastasis (Fig. 1A). On magnetic resonance imaging (MRI), a hypovascular nodule with low signal intensity in T1-weighted images and slightly high signal intensity in T2-weighted images, mimicking cholangiocarcinoma and hepatic metastasis, was observed (Fig. 1B, C). There was no evidence of malignant disease in other organs. Hepatic resection was performed. Nine years after surgery, the patient's course remained uneventful.
PATHOLOGIC FINDINGS
On the cut section, a 1.5×1.5 cm sized, homogeneous, yellow-colored hepatic nodule was observed. The nodule was well-demarcated from surrounding normal liver tissue, but obvious capsulation was not seen (Fig. 1D). Histologically, liver nodule was composed of ischemic-coagulative necrosis with central suspicious parasitic remnant and dense collagenized fibrous tissue (Fig. 2A-C). Outer layer of the lesion was composed of surrounding granulomatous inflammation and peripheral rim-like inflammatory cells infiltration (Fig. 2D). Most inflammatory cells were lymphocytes, and few neutrophils, eosinophils and plasma cells were sprinkled with lymphocytes. Masson's trichrome stain demonstrated the fibrosis of the central necrotic area. Acid-fast bacilli (AFB) stain and periodic acid-Schiff (PAS) stain revealed no bacterial or fungal organism. There was no calcification and evidence of malignancy.

(A) A whole-mount section. (B) Central area composed of suspicious parasitic remnant and surrounding necrotic tissue (H&E stain, ×100). (C) Ischemic-coagulative necrosis with fibrosis (H&E stain, ×400). (D) Peripheral rim-like granulomatous inflammation and inflammatory cells infiltration (H&E stain, ×200).
DISCUSSION
SNN is an unusual hepatic lesion, pathologically characterized by central necrotic core enclosed by a hyalinized fibrotic tissue containing elastic fibers with inflammatory cells.1-4 SNN is usually detected incidentally or during the postmortem autopsy. Most SNNs are single, but may also be multiple.4 Mean diameter of SNN is 2.3 cm and it is found most commonly under the superficial capsule in the right lobe.2,3 It has also been reported that rapid growing of SNN over 8.5 cm in 7 months5 and spontaneous resolution of hepatic SNN after 7 months follow-up.6 SNNs occur in adult-male predominantly (68.6% of cases), and a large proportion of patients (72.5% of cases) show no symptoms, and the others present intermittent abdominal pain, malaise and fever.3 The etiology of SNN is still unclear. Several pathogenetic hypotheses of SNN are suggested: evolution of hepatic hemangioma; lesion of traumatic etiology; and sequelae of previous infection such as parasite.1,4,7,8 In our case, centrally located structure, suspicious for parasitic remnant, supports the possibility of old parasitic infection origin.
Clinically and radiologically, differential diagnosis includes infectious lesions, benign lesions such as hemangioma, focal nodular hyperplasia and adenoma, and malignant lesions such as necrotic metastasis, hepatocellular carcinoma and cholangiocarcinoma. SNN appears as heterogeneous hypoechoic nodule with unclear margins on ultrasonography (US), and shows hypodensity with peripheral enhancement when enhanced on CT scan.3 Above all, solitary necrotic nodule is hard to distinguish from intrahepatic cholangiocarcinoma and necrotic metastasis by US and CT scan.9,10 Furthermore, many patients with SNN show the tendency to be accompanied by primary cancer of other organs.11,12 Percutaneous liver needle biopsy may not be useful for distinguishing SNN from necrotic malignant tumor, if it reveals necrotic tissue only. MRI can be useful for differentiating SNN from other lesions.2,10 According to previous MRI study of thirty-three cases, most SNNs show hypointense in T1-weighted images and variable intensity in T2-weighted images, and none of them show enhancement after contrast administration.2
SNN shares characteristic histologic findings: complete central necrotic core enclosed by a dense hyalinized fibrous capsule with elastic fiber.1-4 Central necrotic core is composed of amorphous eosinophilic granular materials. The central necrotic areas occasionally contain dystrophic calcification, cholesterol clefts, foam cells, and shadows of degenerated cells.7 The peripheral fibrotic portion reveals varying amount of elastic fibers, varying numbers of small arteries and veins, and mononuclear inflammatory cells. Histologic findings may vary depending on the etiology of the SNN. Histologic features, suggesting the evolution of hemangioma, include the presence of feeding vessel and remnant hemangiomatous vascular structures and prominent sclerosis.8 In case of parasitic origin, degenerated larva can be seen in the necrotic center as in our case. Some specific larvae, Clonorchis sinesis,7 filarial nematode,13 Capillaria hepatica,14 and hydatid cyst15 have been detected in SNN. However, the larval structure is commonly too degenerated to identify the exact species. Although SNN shows benign clinical course, complete surgical removal and careful histologic examination are recommended for pathologic confirmation.
Notes
The authors have no conflicts to disclose.
Abbreviations
AFB
acid-fast bacilli
CT
computed tomography
MRI
magnetic resonance imaging
PAS
periodic acid-Schiff
SNN
solitary necrotic nodule
US
ultrasonography