| Clin Mol Hepatol > Volume 18(2); 2012 > Article |
ABSTRACT
Background/Aims
The aim of this study was to analyze the clinical impacts of obesity and hazardous alcohol use on the outcome of entecavir (ETV) therapy in chronic hepatitis B (CHB) patients.
Methods
The medical records of 88 treatment-naïve patients who were diagnosed with CHB and received ETV between March 2007 and September 2009 were analyzed retrospectively. Body mass index (BMI) values and Alcohol Use Disorders Identification Test (AUDIT) scores were obtained at 6 months after the initiation of ETV (0.5 mg daily) treatment.
Results
A BMI of 25 kg/m2 or more was recognized as an indicator of obesity, and a total AUDIT score of 8 or more was recognized as an indicator of hazardous alcohol use. Of the cohort, 24 patients (27.3%) were obese and 17 (19.3%) were hazardous alcohol users. The rate of seroconversion, alanine aminotransferase (ALT) normalization, and hepatitis B virus (HBV)-DNA negativity (<300 copies/mL) at 3, 6, and 12 months of treatment did not differ significantly between the normal-BMI and high-BMI groups. Moreover, the rate of seroconversion and HBV-DNA negativity at 3, 6, and 12 months of treatment did not differ significantly between the nonhazardous and hazardous alcohol users. However, the frequency of ALT normalization at 12 months was significantly lower among hazardous alcohol users (91.5% vs. 70.6%; P=0.033).
Chronic hepatitis B (CHB) is a worldwide health problem. More than 400 million people around the world are chronically infected despite the hepatitis B virus (HBV) vaccination, and those patients have increased risk for complications of CHB, including liver cirrhosis and hepatocellular carcinoma (HCC).1 An increased serum HBV DNA level (>10,000 copies/mL) is a strong risk factor for HCC and liver cirrhosis independent of hepatitis B e-antigen (HBeAg) status and the serum alanine aminotransferase (ALT) level.2,3 Therefore, one of primary goals in treatment of CHB is suppressing HBV replication and reducing complications, such as the development of HCC and cirrhosis.
Entecavir (ETV) is a potent and selective guanosine analogue with a high genetic barrier to resistance and strong suppression of HBV replication. Long-term observation has revealed low rates of resistance in nucleoside-naive patients during 5 years of ETV therapy, with a 5-year cumulative probability of ETV resistance of 1.2%.4 Also, treatment with ETV showed improved histologic, virologic, and biochemical efficacy after 48 weeks of treatment compared to lamivudine (LAM) in HBeAg-positive and HBeAg-negative patients with CHB.5,6 ETV has been recommended as a primary therapy for CHB with potent HBV DNA suppression and low resistance, because the proportion of patients with a documented LAM-resistant mutation increased from 23% in year 1 to 65% in year 5 of treatment with LAM.7 Therefore, ETV is currently used worldwide as primary drug in nucleoside-naïve CHB patients.
However, factors influencing the antiviral effect of ETV have not yet been widely studied. In particular, obesity and alcohol consumption are major factors that have a harmful impact on the liver. A longitudinal cohort study suggested that a higher body mass index (BMI) was associated with transition from healthy HBV carrier state to HCC and liver-related death among men.8 Alcohol is also associated with the development of both alcoholic cirrhosis and HCC.9,10 A report demonstrated that chronic ethanol consumption stimulates HBV gene expression and replication in transgenic mice,11 and one prospective study found that obesity and alcohol synergize to increase the risk of incident HCC in men.12
In this retrospective study, we analyzed the association between alcohol consumption, obesity, and treatment outcomes in naïve CHB patients receiving ETV.
The most of data were extracted from a retrospective cohort study previously performed in our institution.13 Additionally, we retrospectively analyzed the medical records of treatment-naïve patients with CHB who received 0.5 mg ETV once daily for >12 months at our institution between March 2007 and September 2009. ETV was commenced according to the Korean Association for the Study of the Liver.14 Liver cirrhosis was clinically diagnosed (varix, ascites, reasonable result on radiologic method). The exclusion criteria included a co-infection with hepatitis C virus or human immunodeficiency virus, a history of taking other antiviral agents for CHB, decompensated liver cirrhosis, HCC, and a concurrent use of immunosuppressive drugs or corticosteroids and underlying medical diseases accompanied by ascites such as congestive heart failure.
The study protocol was approved by the Institutional Review Board for Human Research at Kangbuk Samsung Hospital. Written, informed consent was obtained from all participating patients.
Serum biochemical parameters, including total bilirubin, ALT, aspartate aminotransferase, gamma glutamyl transpeptidase, alkaline phosphatase, HBeAg, anti-HBeAg, and HBV DNA were measured at baseline. Total bilirubin, ALT, aspartate aminotransferase, albumin, gamma glutamyl transpeptidase, alkaline phosphatase, HBeAg, anti-HBeAg, and HBV DNA were accessed at 3, 6, and 12 months during treatment. Serum levels of HBV DNA were quantified with a real-time polymerase chain reaction (PCR) assay by a Cobas TaqMan 48 analyzer (Roche Molecular Systems, Branchburg, NJ, USA). Treatment response was evaluated at 3, 6, and 12 months after initiation of ETV. Complete virologic response (CVR) was defined as an HBV DNA level by a real-time PCR assay of <300 copies/mL, and biochemical response (BR) was defined as normalization of the ALT level (ALT level ≤1×the upper normal limit) in subjects with an abnormal baseline ALT level at 6 months after initiation of ETV treatment. Non virologic response was defined as an HBV DNA decline of <2 log10 copies/mL at 6 months after initiation of ETV treatment. Virologic breakthrough was defined as an increase in HBV DNA levels to >1 log10 copies/mL above the treatment nadir during the follow-up period.
Weight and height were measured by a trained nurse at 6 months after initiation of ETV treatment. BMI was calculated using weight divided by height squared (kg/m2), and subjects were categorized into the normal group (BMI <25 kg/m2) and the high BMI group (BMI ≥25 kg/m2).
At 6 months after initiation of treatment, we interviewed enrolled patients using the alcohol use disorder identification test (AUDIT) questionnaire of the World Health Organization (WHO) to investigate the status of alcohol intake.15 The AUDIT questionnaire was suggested to identify individuals with an early alcohol disorder in primary care,16 and recent studies reported the use of AUDIT as a proper tool of screening for the various spectrum of alcohol use disorders. A total score of 8 or more is recommended as an indicator of hazardous alcohol use according to scoring protocol.15 AUDIT scores of each patient were obtained and subjects were divided into the hazardous alcohol group (AUDIT score ≥8) and the non-hazardous alcohol group (AUDIT score <8). In each group, treatment outcomes were analyzed.
Serum HBV DNA levels were converted to a common logarithm. The characteristics of total enrolled patients were given as the mean±SD, where applicable. To summarize the continuous variables, we used medians and IQR (Interqartile range). The chi-square test or Fisher's exact test and the Student t-test were used to compare the categorical and continuous variables, respectively. We used the chi-square test or Fisher's exact test to compare treatment outcomes according to BMI and alcohol use. To investigate the independent predictors for CVR at 12 months, univariate logistic regression was performed. And a multivariate logistic regression was carried out for significant variables. Age, sex, HBeAg, ALT, HBV DNA, liver cirrhosis, BMI and AUDIT score were included in multivariate analysis. They were described on the footnote of Table 3. A P-value less than 0.05 was considered to be statistically significant. SPSS version 18.0 (SPSS, Inc., Chicago, IL, USA) was the software used for all statistical analyses.
A total of 88 patients were enrolled. The baseline characteristics of the patients are shown in Table 1. The mean age was 45.6 years, and 60 patients (68.2%) were male, 44 patients (50.0%) were HBeAg-positive, and 19 cases (21.6%) were patients with liver cirrhosis. The median or mean baseline values of ALT, log10 HBV DNA, BMI and AUDIT score were 107 IU/L, 6.3 log10 copies/mL, 23.3 kg/m2 and 1.5 respectively.
The baseline characteristics of the two groups according to BMI and alcohol use are shown in Table 2. Twenty-four patients (27.3%) were enrolled in the high BMI group. There were no significant differences in baseline characteristics between the normal and high BMI groups except sex. As expected, the number of men was significantly higher than that of women in the high BMI group (91.7% vs. 59.4%, P=0.004). Seventeen patients (19.3%) consumed a hazardous level of alcohol. The baseline characteristics of two groups according to alcohol use are described in Table 2. Significantly more men were included in the hazardous alcohol use group (94.1% vs. 62.0%, P=0.011).
At 3, 6, and 12 months after initiation of ETV treatment, CVR was achieved in 41 (46.6%), 61 (69.3%), and 73 (83.0%) patients. BR was observed in 63 (71.6%), 76 (86.4%), and 81 (92.0%) patients at 3, 6, and 12 months after initiation of ETV treatment (from a total of 88 patients who had elevated baseline ALT level). 9 (20.5%), 11 (25%), and 15 patients (34.1%) achieved HBeAg seroconversion at 3, 6, and 12 months after initiation of ETV treatment in HBeAg positive (n=44) patients. CVR was achieved in 9 (20.5%), 21 (47.7%), and 31 patients (70.5%) in the HBeAg positive group (n=44) and in 32 (72.7%), 40 (90.9%), and 42 patients (95.5%) in the HBeAg negative group. Non virologic response and virologic breakthrough did not occur during the study period.
Predictors for CVR at 12 months after initiation of ETV therapy were investigated in all patients (Table 3). Among the baseline characteristics, univariate analysis showed that an absence of HBeAg (P=0.006) and a low HBV DNA level at baseline (P=0.002) were significantly associated with CVR at 12 months. In multivariate analysis, a high ALT level (P=0.029, OR=1.015, 95% CI 1.002-1.029) and a low HBV DNA level at baseline (P=0.026, OR=0.495, 95% CI 0.266-0.919) was significant predictor of CVR.
A total of 83.3% (20/24) of patients with high BMI and 64.1% (41/64) of patients with normal BMI achieved HBV DNA levels <300 copies/mL by PCR assay at 6 months after inititiation of ETV treatment. By 12 months after the initiation of of ETV treatment, 87.5% (21/24) of patients with high BMI and 81.3% (52/64) of patients with normal BMI achieved HBV DNA levels <300 copies/mL by PCR assay (Fig. 1). There were no significant differences in the CVR rate between the high and normal BMI groups (P=0.081 at 6 months and P=0.751 at 12 months).
A total of 70.6% (12/17) of hazardous alcohol use group patients and 69.0% (49/71) of non hazardous alcohol use patients achieved HBV DNA levels <300 copies/mL by PCR assay at 6 months after initiation of ETV treatment. By 12 months after initiation of of ETV treatment, 82.4% (14/17) of hazardous alcohol use group patients and 83.1% (59/71) of non hazardous alcohol use patients achieved HBV DNA levels <300 copies/mL by PCR assay (Fig. 2). Our data showed no significant differences in CVR rate between the hazardous and non hazardous alcohol use groups (P=0.899 at 6 months and P=1.000 at 12 months).
A total of 75% (18/24) of patients with high BMI and 90.6% (58/64) of patients with normal BMI achieved BR at 6 months after initiation of ETV treatment. By 12 months after initiation of ETV treatment, 75% (18/24) of patients with high BMI and 92.2% (59/64) of patients with normal BMI achieved BR (Fig. 1). High BMI did not significantly affect the BR rate in this study (P=0.081 at 6 months and P=0.063 at 12 months).
A total of 82.4% (14/17) of hazardous alcohol use patients and 87.3% (62/71) of non hazardous alcohol use patients achieved BR at 6 months after initiation of ETV treatment. By 12 months after initiation of ETV therapy, the proportion of patients who achieved BR increased to 91.5% (65/71) for the non hazardous alcohol use group and decreased to 70.6% (12/17) for the hazardous alcohol use group (Fig. 2). Hazardous alcohol consumption resulted in a significantly lower cumulative BR rate by 12 month after initiation of ETV therapy (P=0.694 at 6 months and P=0.033 at 12 months).
Seroconversion occurred in 25% (2/8) at 6 months and 25% (2/8) at 12 months of the HBeAg positive patients with high BMI (Fig. 1). Those rates were similar to those with normal BMI (25% [9/36] and 36.1% [13/36], respectively; P=1.000 for the comparison of seroconversion rate at 6 months between the groups and P=0.695 for the comparison of seroconversion rate at 12 months between the groups).
Seroconversion occurred in 20% (2/10) at 6 months and 20% (2/10) at 12 months of the HBeAg positive patients with hazardous alcohol use (Fig. 2). Of the HBeAg positive patients with non hazardous alcohol use, seroconversion occurred in 26.5% (9/34) at 6 months and 38.2% (13/34) at 12 months (P=1.000 for the comparison of seroconversion rate at 6 months between the groups and P=0.452 for the comparison of seroconversion rate at 12 months between the groups). A hazardous level of alcohol and high BMI did not disturb the seroconversion rate in patients receiving ETV.
Although the BR rate at 12 months after initiation of ETV treatment was significantly lower in the hazardous alcohol use group, Our data demonstrated no significant differences in the serial treatment outcome of ETV in treatment-naïve CHB patients between two groups according to alcohol consumtion and BMI on the condition that there were no significant differences in HBeAg negativity and level of HBV DNA at baseline between two groups. Recent investigations reported low baseline HBV DNA level, absence of HBeAg, low baseline quantitative HBsAg titer, and high ALT level as predictors of the virologic response to ETV.17,18 Our data also revealed that an absence of HBeAg and a low HBV DNA level at baseline were significant predictors of the CVR at 12 months in a univariate analysis and a high ALT level and low HBV DNA at baseline were significant predictors in multivariate analysis. The level of HBsAg titer could not be obtained because quantitative assay of HBsAg level was not available in our institution during follow up period.
As predictors of treatment response, obesity and alcohol drinking on ETV efficacy for CHB patients had not yet been studied. Alcohol and obesity are well known for their noxious effect on the liver and expected to have the negative effect on ETV efficacy. However, contrary to our expectations, ETV showed the satisfactory antiviral effect in the obese and hazardous alcohol use patients with CHB.
This current study provides data on the strong antiviral effect of ETV in nucleoside-naïve CHB patients and demonstrates that CVR, normalization of ALT, and seroconversion were 82.9%, 87.5%, and 34.0% at 12 months after initiation of ETV treatment. CVR at 12 months was 70.4% in HBeAg positive patients and 95.4% in HBeAg negative patients. HBeAg negative patients showed more favorable treatment results than HBeAg positive patients. Although our data demonstrated a higher seroconversion rate than other reports, it had a limitation, because the numbers of HBeAg positive patients (n=44) were small.
Previous reports found a negative impact of obesity and steatosis on the treatment response rates in patients with chronic hepatitis C (CHC) undergoing peginterferon treatment.19-21 In contrast, the clinical impact of obesity in CHB patients receiving oral nucleostide analogues remains largely unknown. Cindoruk et al investigated the relevance of hepatic steatosis in CHB patients treated with LAM, which was known to be a less potent agent.22 In our study, significant differences in CVR, BR, and seroconversion were not detected between the high BMI and normal BMI groups. Obesity as an inflammatory condition can indirectly interfere with the immunomodulatory effects of peginterferon by several mechanisms in CHC patients.23 It is thought that accumulation of fat material in the parenchyma of liver leads to liver tissue that is more susceptible to harmful stimuli. For this reason, obese patients are expected to have a poor response to antiviral therapy. However, in this analysis, obesity did not disturb the treatment outcomes of CHB patients receiving ETV.
The hazardous alcohol use group showed similar CVR and seroconversion rate compared to the non hazardous alcohol use group. Several reports indicated that chronic ethanol consumption substantially alters the cellular immune responses to human viral structural protein, and this effect may cause the persistence of HBV infection and increased viral gene expression.24 In this study, significant differences in HBV DNA reduction and seroconversion were not noted between the hazardous and non-hazardous alcohol use groups. ETV reduced the HBV DNA level and led to seroconversion regardless of hazardous level of alcohol consumption. On the other hand, BR was significantly lower in the hazardous alcohol use group at 12 months after initiation of ETV treatment. Chronic alcohol consumption is one of the major causes of liver enzyme elevation25 and seems to influence BR rate of alcohol use patients taking ETV.
Our study is the first report to evaluate the clinical impact of alcohol use and obesity on the efficacy of ETV. The data in this study are valuable, because serial treatment outcomes were analyzed in obese or hazardous alcohol use patients treated with ETV as a first-line therapy for CHB. We found almost no reports regarding the effect of alcohol on ETV treatment, perhaps because CHB patients are almost always advised to quit drinking during treatment. In our report, before antiviral therapy, CHB patients were strongly encouraged to stop drinking alcohol by their attending physicians and nurse. However, some of them continued drinking during treatment despite being cautioned by their physicians, and a minority of them showed alcohol dependence. Those patients were enrolled in this study.
In the present study, some limitations exist in evaluating the effect of obesity and alcohol on ETV efficacy. First, this report is a retrospective cohort study performed in a single institution for a single ethnic group of patients. Second, there were small numbers of patients enrolled in the hazardous alcohol group and a small number of patients with high grade obesity, because people in South Korea have a lower frequency of obesity than those in the Western population. Third, in investigating alcohol intake, this study depended on an interview with patients or their families. Although interviews were performed by a trained individual, this method may lead to an inaccurate estimate of alcohol intake. This is likely to underestimate the amount of alcohol use and can potentially lead to misclassification bias. Finally, we were not able to evaluate the development of resistance to ETV because of the short follow-up period.
In conclusion, obesity and hazardous alcohol drinking had no significant impact on the outcome of ETV in treatment-naïve CHB patients. Although the BR rate at 12 months after initiation of ETV treatment was significantly lower in the hazardous alcohol use group, ETV showed the satisfactory treatment response even in the obese and hazardous alcohol use patients. A further prospective study for large number of patients will be necessary to validate the finding of this present study.
Abbreviations
ALT
alanine aminotransferase
AUDIT
alcohol use disorder identification test
BR
biochemical response
CHB
chronic hepatitis B
CHC
chronic hepatitis C
CVR
complete virologic response
ETV
Entecavir
HBV
hepatitis B virus
HCC
hepatocellular carcinoma
HBeAg
hepatitis B e-antigen
LAM
lamivudine
PCR
polymerase chain reaction
WHO
World Health Organization
REFERENCES
1. McMahon BJ. Epidemiology and natural history of hepatitis B. Semin Liver Dis 2005;25(Suppl 1):3-8. 16103976.



2. Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology 2006;130:678-686. 16530509.


3. Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006;295:65-73. 16391218.


4. Tenney DJ, Rose RE, Baldick CJ, Pokornowski KA, Eggers BJ, Fang J, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naïve patients is rare through 5 years of therapy. Hepatology 2009;49:1503-1514. 19280622.


5. Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med 2006;354:1001-1010. 16525137.


6. Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med 2006;354:1011-1020. 16525138.


7. Lok AS, Lai CL, Leung N, Yao GB, Cui ZY, Schiff ER, et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology 2003;125:1714-1722. 14724824.


8. Yu MW, Shih WL, Lin CL, Liu CJ, Jian JW, Tsai KS, et al. Body-mass index and progression of hepatitis B: a population-based cohort study in men. J Clin Oncol 2008;26:5576-5582. 18955457.


9. Donato F, Tagger A, Gelatti U, Parrinello G, Boffetta P, Albertini A, et al. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol 2002;155:323-331. 11836196.



10. Hassan MM, Hwang LY, Hatten CJ, Swaim M, Li D, Abbruzzese JL, et al. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology 2002;36:1206-1213. 12395331.


11. Larkin J, Clayton MM, Liu J, Feitelson MA. Chronic ethanol consumption stimulates hepatitis B virus gene expression and replication in transgenic mice. Hepatology 2001;34:792-797. 11584377.


12. Loomba R, Yang HI, Su J, Brenner D, Iloeje U, Chen CJ. Obesity and alcohol synergize to increase the risk of incident hepatocellular carcinoma in men. Clin Gastroenterol Hepatol 2010;8:891-898. 898.e1-898.e2. 20621202.


13. Kim HJ, Park DI, Park JH, Cho YK, Sohn CI, Jeon WK, et al. Comparison between clevudine and entecavir treatment for antiviral-naïve patients with chronic hepatitis B. Liver Int 2010;30:834-840. 20408946.


14. Lee KS, Kim DJ. Korean Association for the Study of the Liver Guideline Committee. Management of chronic hepatitis B. Korean J Hepatol 2007;13:447-488. 19054901.


15. Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. The Alcohol Use Disorders Identification Test: Guideline for use in Primary Care. 2001. Geneva: World Health Organization; p. 1-41.
16. Skipsey K, Burleson JA, Kranzler HR. Utility of the AUDIT for identification of hazardous or harmful drinking in drug-dependent patients. Drug Alcohol Depend 1997;45:157-163. 9179517.


17. Lee JM, Ahn SH, Kim HS, Park H, Chang HY, Kim do Y, et al. Quantitative hepatitis B surface antigen and hepatitis B e antigen titers in prediction of treatment response to entecavir. Hepatology 2011;53:1486-1493. 21520167.


18. Myung HJ, Jeong SH, Kim JW, Kim HS, Jang JH, Lee DH, et al. Efficacy and predictors of the virologic response to entecavir therapy in nucleoside-naive patients with chronic hepatitis B. Korean J Hepatol 2010;16:57-65. 20375643.


19. Harrison SA, Brunt EM, Qazi RA, Oliver DA, Neuschwander-Tetri BA, Di Bisceglie AM, et al. Effect of significant histologic steatosis or steatohepatitis on response to antiviral therapy in patients with chronic hepatitis C. Clin Gastroenterol Hepatol 2005;3:604-609. 15952103.


20. Thomopoulos KC, Theocharis GJ, Tsamantas AC, Siagris D, Dimitropoulou D, Gogos CA, et al. Liver steatosis is an independent risk factor for treatment failure in patients with chronic hepatitis C. Eur J Gastroenterol Hepatol 2005;17:149-153. 15674091.


21. Patton HM, Patel K, Behling C, Bylund D, Blatt LM, Vallée M, et al. The impact of steatosis on disease progression and early and sustained treatment response in chronic hepatitis C patients. J Hepatol 2004;40:484-490. 15123364.


22. Cindoruk M, Karakan T, Unal S. Hepatic steatosis has no impact on the outcome of treatment in patients with chronic hepatitis B infection. J Clin Gastroenterol 2007;41:513-517. 17450036.


23. Charlton MR, Pockros PJ, Harrison SA. Impact of obesity on treatment of chronic hepatitis C. Hepatology 2006;43:1177-1186. 16729327.


Figure 1
Comparison of the treatment response rate between the normal-BMI and high-BMI patients. The rate of complete viral response (CVR) was 42.2% in the normal BMI group vs. 58.3% in high BMI group at 3 months (P=0.176), 64.1% vs. 83.3% at 6 months (P=0.081), and 81.3% vs. 87.5% at 12 months (P=0.751). The biochemical response (BR) rate was 76.6% in the normal BMI group vs. 58.3% in the high BMI group at 3 months (P=0.091), 90.6% vs. 75.0% at 6 months (P=0.081), and 92.2% vs. 75.0% at 12 months (P=0.063). The seroconversion rate was 22.2% in the normal BMI group vs. 12.5% in the high BMI group at 3 months (P=1.000). 25.0% vs. 25.0% at 6 months (P=1.000), and 36.1% vs. 25.0% at 12 months (P=0.695).
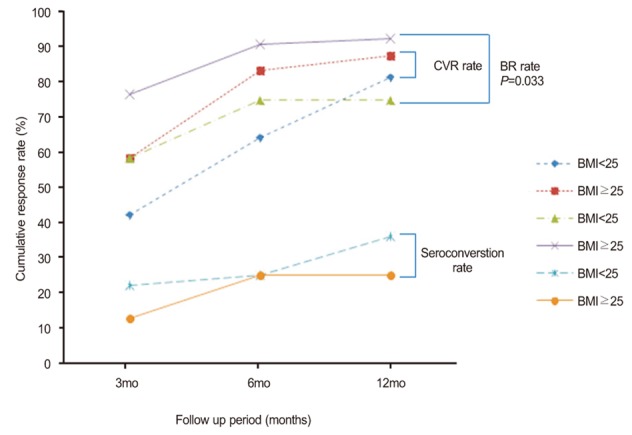
Figure 2
Comparison of the treatment response rate between the nonhazardous alcohol use group and the hazardous alcohol use group. The CVR rate was 46.5% in the nonhazardous alcohol use group vs. 47.1% in the hazardous alcohol use group at 3 months (P=0.966), 69.0% vs. 70.6% at 6 months P=0.899), and 83.1% vs. 82.4% at 12 months (P=1.000). The BR rate was 71.8% in the nonhazardous alcohol use group vs. 70.6% in the hazardous alcohol use group at 3 months (P=1.000), 87.3% vs. 82.4% at 6 months (P=0.694), and 91.5% vs. 70.6% at 12 months (P=0.033). The seroconversion rate was 23.5% in the nonhazardous alcohol use group vs. 10.0% in the hazardous alcohol use group at 3months (P=0.659), 26.5% vs. 20.0% at 6 months (P=1.000), and 38.2% vs. 20.0% at 12 months (P=0.452).
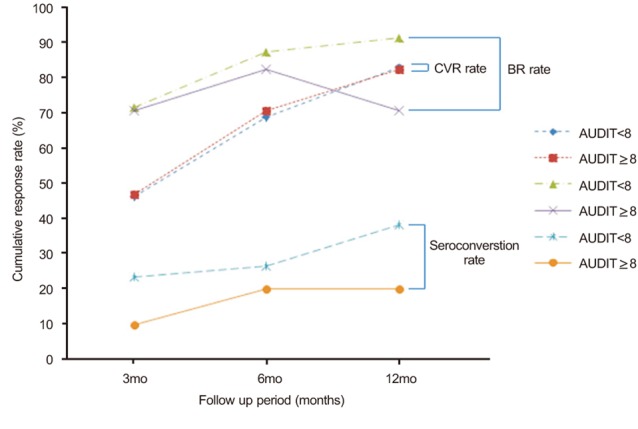
Table 1.
Baseline and follow up characteristics of enrolled patients
Volues are presented as n (%) unless otherwise indicated.
SD, standard deviation; IQR, interqartile range; HBeAg, hepatitis B e antigen; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ETV, entecavir; HBV, hepatitis B virus; BMI, body mass index; AUDIT, alcohol use disorder identification test.
Table 2.
Comparision of baseline characteristics between two groups according to alcohol use and BMI
| Characteristics | Normal BMI (n=64) (BMI <25) | High BMI (n=24) (BMI ≥25) | P-value | Non hazardous drinker (n=71) (AUDIT score <8) | Hazardous drinker (n=17) (AUDIT score ≥8) | P-value |
|---|---|---|---|---|---|---|
| Age (yr, mean±SD) | 46.1±11.6 | 44.3±9.1 | 0.502* | 46.0±11.7 | 43.9±6.9 | 0.484* |
| Male | 38 (59.4%) | 22 (91.7%) | 0.004† | 44 (62.0%) | 16 (94.1%) | 0.011† |
| HBeAg-positive | 36 (56.3%) | 8 (33.3%) | 0.056† | 34 (47.9%) | 10 (58.8%) | 0.418† |
| Baseline serum ALT (IU/L, median [IQR]) | 107 (64.3-225.8) | 103 (51.8-173.3) | 0.536* | 108 (64-195) | 101 (58-250) | 0.983* |
| Baseline serum HBV DNA (log10 copies/mL, mean±SD) | 6.43±1.442 | 5.89±1.485 | 0.121§ | 6.35±1.409 | 5.99±1.698 | 0.360§ |
| Cirrhosis | 13 (20.3%) | 6 (25.0%) | 0.634‡ | 14 (19.7%) | 5 (29.4%) | 0.511‡ |
| BMI (kg/m2, (median [IQR]) | 21.9 (20.5-23.6) | 26.4 (25.5-27.6) | <0.001* | 23.4 (20.8-25.1) | 23.3 (21.6-26.4) | 0.303* |
| Audit score (median, IQR) | 1 (0-5) | 2 (0-4.75) | 0.926* | 0 (0-3) | 11 (9.5–14.5) | <0.001* |
Table 3.
Results of univariate and multivariate analysis of the predictive factors for CVR at 12 months to ETV therapy in treatment naïve CHB patients
| Baseline parameters | CVR (n=73) | Non CVR (n=15) | Univariate P-value | OR (95% CI) | Multivariate P-value | OR (95% CI) |
|---|---|---|---|---|---|---|
| Age (yr, mean±SD) | 45.9±11.1 | 44.1±10.4 | 0.561* | 1.016 (0.964-1.070) | 0.378* | 1.032 (0.962-1.108) |
| Male | 48 (65.8%) | 12 (80%) | 0.288* | 2.083 (0.538-8.071) | 0.355* | 2.442 (0.368-16.205) |
| HBeAg negativity | 42 (57.5%) | 2 (13.3%) | 0.006* | 0.114 (0.024-0.540) | 0.091* | 0.187 (0.027-1.309) |
| ALT (IU/L, median [IQR]) | 120 (64.5-250) | 92 (62-106) | 0.057* | 1.008 (1.000-1.017) | 0.029*† | 1.015 (1.002-1.029) |
| HBV DNA (log10 copies/mL, mean±SD) | 6.0±1.4 | 7.4±1.2 | 0.002* | 0.496 (0.316-0.778) | 0.026*‡ | 0.495 (0.266-0.919) |
| Liver cirrhoisis | 18 (24.7%) | 1 (6.7%) | 0.155* | 4.582 (0.563-37.319) | 0.234* | 4.104 (0.400-42.071) |
| BMI ≥25 | 21 (28.8%) | 3 (20%) | 0.490* | 1.615 (0.413-6.312) | 0.510* | 1.912 (0.278-13.129) |
| AUDIT score ≥8 | 14 (19.2%) | 3 (20%) | 0.941* | 0.949 (0.236-3.822) | 0.759* | 1.373 (0.181-10.402) |



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




