| Clin Mol Hepatol > Volume 29(3); 2023 > Article |
|
ABSTRACT
Background/Aims
Methods
Results
ACKNOWLEDGMENTS
FOOTNOTES
SUPPLEMENTAL MATERIAL
Supplementary┬ĀTable┬Ā2.
Supplementary┬ĀTable┬Ā3.
Supplementary┬ĀTable┬Ā4.
Supplementary┬ĀTable┬Ā5.
Supplementary┬ĀTable┬Ā6.
Supplementary┬ĀTable┬Ā8.
Supplementary┬ĀFigure┬Ā1.
Supplementary┬ĀFigure┬Ā2.
Supplementary┬ĀFigure┬Ā3.
Supplementary┬ĀFigure┬Ā4.
Supplementary┬ĀFigure┬Ā5.
Supplementary┬ĀFigure┬Ā6.
Supplementary┬ĀFigure┬Ā7.
Supplementary┬ĀFigure┬Ā8.
Supplementary┬ĀFigure┬Ā9.
Supplementary┬ĀFigure┬Ā10.
Supplementary┬ĀFigure┬Ā11.
Supplementary┬ĀFigure┬Ā12.
Supplementary┬ĀFigure┬Ā13.
Supplementary┬ĀFigure┬Ā14.
Supplementary┬ĀFigure┬Ā15.
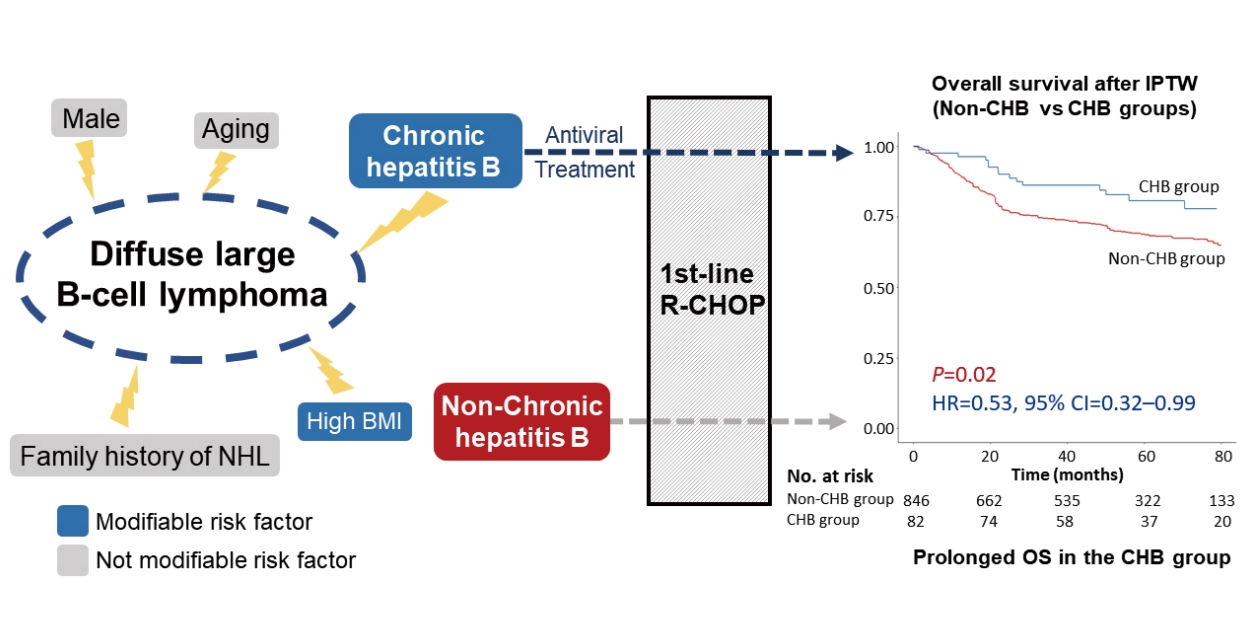
Figure┬Ā1.
Figure┬Ā2.
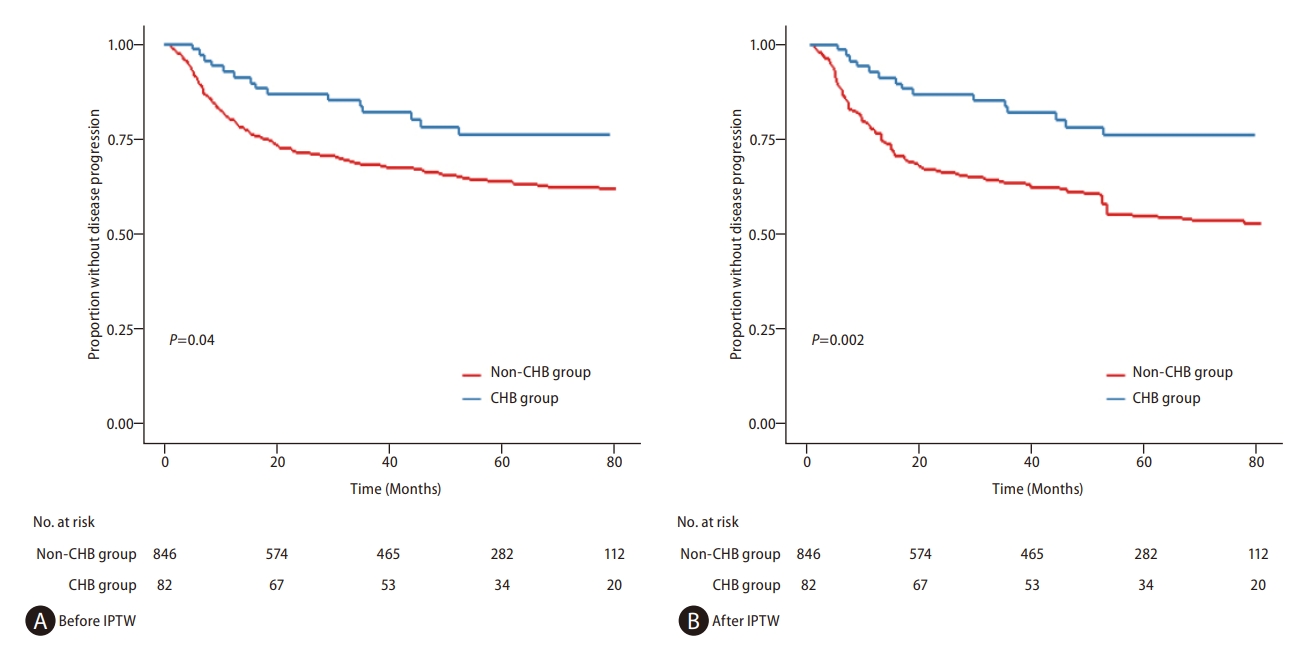
Figure┬Ā3.

Table┬Ā1.
| Variables |
Before IPTW |
After IPTW |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| CHB group (n=82) | Non-CHB group (n=846) | P-value | SMD | CHB group (n=82) | Non-CHB group (n=846) | P-value | SMD | ||
| Age (yr) | 56.5 (48.0ŌĆō67.0) | 63.0 (53.0ŌĆō71.0) | 0.002 | 0.37 | 56.0 (48.0ŌĆō66.0) | 58.0 (49.0ŌĆō68.0) | 0.75 | <0.01 | |
| Sex | 0.20 | 0.16 | 0.95 | <0.01 | |||||
| Female | 29 (35.4) | 367 (43.4) | 29 (35.4) | 303 (35.8) | |||||
| Male | 53 (64.6) | 479 (56.6) | 53 (64.6) | 543 (64.2) | |||||
| Ann Arbor classification | 0.07 | 0.27 | 0.28 | 0.02 | |||||
| Stage I | 8 (9.8) | 133 (15.7) | 8 (9.8) | 102 (12.1) | |||||
| Stage II | 17 (20.7) | 234 (27.7) | 17 (20.7) | 163 (19.3) | |||||
| Stage III | 16 (19.5) | 100 (11.8) | 16 (19.5) | 100 (11.8) | |||||
| Stage IV | 41 (50.0) | 379 (44.8) | 41 (50.0) | 481 (56.8) | |||||
| BM involvement | 0.20 | 0.07 | 0.91 | 0.05 | |||||
| Present | 19 (23.2) | 135 (16.0) | 19 (23.2) | 196 (23.2) | |||||
| Absent | 59 (72.0) | 649 (76.7) | 59 (72.0) | 618 (73.0) | |||||
| Unknown | 4 (4.9) | 62 (7.3) | 4 (4.9) | 32 (3.8) | |||||
| Body mass index (kg/m2) | 24.6 (22.1ŌĆō26.3) | 23.9 (21.2ŌĆō26.9) | 0.54 | 0.02 | 24.0 (22.0ŌĆō26.0) | 24.0 (21.0ŌĆō26.0) | 0.74 | 0.04 | |
| ChildŌĆōPugh score | 0.60 | 0.05 | 0.08 | 0.04 | |||||
| A5 | 67 (81.7) | 619 (73.2) | 67 (81.7) | 594 (70.2) | |||||
| A6 | 13 (15.9) | 169 (20.0) | 13 (15.9) | 179 (21.2) | |||||
| B7 | 2 (2.4) | 46 (5.4) | 2 (2.4) | 63 (7.4) | |||||
| B8 | 0 (0.0) | 2 (0.4) | 0 (0.0) | 3 (0.3) | |||||
| B9 | 0 (0.0) | 2 (0.4) | 0 (0.0) | 7 (0.9) | |||||
| Extranodal involvement | 0.46 | 0.10 | 0.98 | <0.01 | |||||
| Present | 62 (75.6) | 601 (71.0) | 62 (75.6) | 640 (75.7) | |||||
| Absent | 20 (24.4) | 245 (29.0) | 20 (24.3) | 206 (24.3) | |||||
| FIB-4 | 1.6 (1.0ŌĆō2.4) | 1.3 (0.9ŌĆō2.1) | 0.07 | 0.13 | 2.0 (1.0ŌĆō2.0) | 1.0 (0.8ŌĆō2.0) | 0.38 | 0.11 | |
| Glomerular filtration rate (mL/min/1.73m┬▓) | 0.94 | 0.03 | 0.74 | 0.04 | |||||
| <60 | 9 (11.0) | 85 (10.0) | 9 (11.0) | 83 (9.8) | |||||
| Ōēź60 | 73 (89.0) | 761 (90.0) | 73 (89.0) | 763 (90.2) | |||||
| HBeAg | <0.001 | 0.67 | <0.001 | 0.67 | |||||
| Positive | 15 (18.3) | 0 (0.0) | 15 (18.3) | 0 (0.0) | |||||
| Negative | 67 (81.7) | 846 (100.0) | 67 (81.7) | 846 (100.0) | |||||
| Hemoglobin (g/dL) | 13.1 (12.1ŌĆō14.8) | 12.9 (11.6ŌĆō14.1) | 0.25 | 0.10 | 13.0 (12.0ŌĆō15.0) | 13.0 (11.0ŌĆō14.0) | 0.39 | 0.04 | |
| IPI risk | 0.01 | 0.10 | 0.33 | 0.03 | |||||
| Low | 34 (41.5) | 231 (27.3) | 31 (37.8) | 407 (48.1) | |||||
| Low-intermediate | 31 (37.8) | 474 (56.0) | 34 (41.5) | 272 (32.2) | |||||
| High-intermediate | 16 (19.5) | 135 (16.0) | 16 (19.5) | 159 (18.8) | |||||
| High | 1 (1.2) | 6 (0.7) | 1 (1.2) | 8 (1.0) | |||||
| LDH | 0.05 | 0.24 | 0.97 | <0.01 | |||||
| Elevated | 29 (35.4) | 399 (52.8) | 29 (35.4) | 301 (35.6) | |||||
| Normal | 53 (64.6) | 447 (52.8) | 53 (64.6) | 545 (64.4) | |||||
| Platelet (10┬│/╬╝L) | 212 (158ŌĆō268) | 247 (191ŌĆō301) | 0.003 | 0.17 | 211 (158ŌĆō265) | 244 (174ŌĆō304) | 0.33 | 0.13 | |
| Subtype of DLBCL | 0.76 | 0.10 | 0.97 | <0.01 | |||||
| GCB | 29 (35.4) | 311 (36.8) | 29 (35.4) | 131 (33.9) | |||||
| Non-GCB | 50 (61.0) | 487 (57.6) | 50 (61.0) | 163 (62.4) | |||||
| Unknown | 3 (3.7) | 48 (5.7) | 3 (3.7) | 10 (3.7) | |||||
| Total bilirubin (mg/dL) | 0.7 (0.5ŌĆō1.0) | 0.6 (0.5ŌĆō0.8) | 0.014 | 0.03 | 0.7 (0.5ŌĆō1.0) | 0.6 (0.5ŌĆō0.9) | 0.15 | 0.02 | |
| Underlying liver status | 0.03 | 0.24 | 0.55 | 0.08 | |||||
| No cirrhosis | 72 (87.8) | 800 (94.6) | 72 (87.8) | 764 (90.3) | |||||
| Cirrhosis | 10 (12.2) | 46 (5.4) | 10 (12.2) | 82 (9.7) | |||||
| Antiviral agents* | |||||||||
| Entecavir | 49 (59.8) | 106 (12.5) | 49 (59.8) | 94 (11.1) | |||||
| Tenofovir | 16 (19.5) | 59 (7.0) | 16 (19.5) | 45 (5.3) | |||||
| Telbivudine | 11 (13.4) | 28 (3.3) | 11 (13.4) | 48 (5.7) | |||||
| Lamivudine | 6 (7.3) | 66 (7.8) | 6 (7.3) | 58 (6.8) | |||||
| Besifovir | 0 (0.0) | 1 (0.1) | 0 (0.0) | 2 (0.2) | |||||
| None | 0 (0.0) | 586 (69.3) | 0 (0.0) | 602 (71.1) | |||||
Continuous variables are presented as the median (interquartile range) and categorical variables are expressed as number (%).
BM, bone marrow; CHB, chronic hepatitis B; DLBCL, diffuse large B-cell lymphoma; FIB-4, fibrosis-4; GCB, germinal center B cell; HBV, hepatitis B virus; HBeAg, hepatitis B e antigen; IPI, International Prognostic Index; IPTW, inverse probability of treatment weighting; LDH, lactate dehydrogenase; SMD, standardized mean difference.
Table 2.
BM, bone marrow; CHB, chronic hepatitis B; CI, confidence interval; DLBCL, diffuse large B-cell lymphoma; GCB, germinal center B cell; HBV, hepatitis B virus; HR, hazard ratio; IPI, International Prognostic Index; IPTW, inverse probability of treatment weighting; LDH, lactate dehydrogenase; TTP, time-to-progression.
Table 3.
BM, bone marrow; CHB, chronic hepatitis B; CI, confidence interval; DLBCL, diffuse large B-cell lymphoma; GCB, germinal center B cell; HBV, hepatitis B virus; HR, hazard ratio; IPI, International Prognostic Index; IPTW, inverse probability of treatment weighting; LDH, lactate dehydrogenase; OS, overall survival.
Abbreviations
REFERENCES
- TOOLS
-
 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link XML Download
XML Download Full text via DOI
Full text via DOI-
 Download Citation
Download Citation
 Supplement1
Supplement1 Supplement2
Supplement2 Supplement3
Supplement3 Supplement4
Supplement4 Supplement5
Supplement5 Supplement6
Supplement6 Supplement7
Supplement7 Supplement8
Supplement8 Supplement9
Supplement9 Supplement10
Supplement10 Supplement11
Supplement11 Supplement12
Supplement12 Supplement13
Supplement13 Supplement14
Supplement14 Supplement15
Supplement15 Supplement16
Supplement16 Supplement17
Supplement17 Supplement18
Supplement18 Supplement19
Supplement19 Supplement20
Supplement20 Supplement21
Supplement21 Supplement22
Supplement22 Supplement23
Supplement23 Supplement24
Supplement24 Supplement25
Supplement25 Supplement26
Supplement26 Print
Print-
Share :



-
METRICS

- ORCID iDs
-
Tae Min Kim

https://orcid.org/0000-0001-6145-4426Jeong-Hoon Lee

https://orcid.org/0000-0002-0315-2080 - Related articles
-
Treatment of Chronic Hepatitis B ; Dose and Treatment Duration of Regimen2005 March;11(1)