Overview of Asian clinical practice guidelines for the management of hepatocellular carcinoma: An Asian perspective comparison
Article information
Abstract
Hepatocellular carcinoma (HCC) is highly prevalent and the third most common cause of cancer-related death in Asia. In contrast to the West, the main etiology of HCC in many Asian countries except Japan is chronic hepatitis B virus infection. Differences in the major causes of HCC lead to significant clinical and treatment differences. This review summarizes and compares guidelines on managing HCC from China, Hong Kong, Taiwan, Japan, and South Korea. From oncology and socio-economic perspectives, factors such as underlying diseases, staging methods, government policies, insurance coverage, and medical resources contribute to varying treatment strategies among countries. Furthermore, the differences in each guideline are fundamentally caused by the lack of incontrovertible medical evidence, and even existing results of clinical trials can be interpreted differently. This review will provide a complete overview of the current Asian guidelines for HCC in recommendations and in practice.
INTRODUCTION
Hepatocellular carcinoma (HCC) is a highly prevalent cancer in Asia when compared to the West and is the third most common cause of cancer-related death in the Asia-Pacific region [1,2]. Despite a hepatitis B virus (HBV) vaccination program, the most prevalent etiology of HCC in Asia except for Japan is still chronic hepatitis B infection, followed by hepatitis C virus (HCV) infection [3].
In contrast to other malignancies, HCC has a high-risk factor for occurrence, making surveillance testing important. Due to the difficulty and risk of tissue diagnosis, it is often confirmed through imaging tests alone. Most patients have chronic liver disease, so treatment strategies are determined based on their underlying conditions, such as liver disease. In addition, liver transplantation and transarterial chemoembolization (TACE) show big differences in treatment compared to other cancers. Because the underlying liver disease remains even after radical surgery, the recurrence rate of HCC is higher than in other cancers, which leads to consideration for subsequent treatment and a multidisciplinary approach.
After the Barcelona Clinic Liver Cancer group developed combined guidelines for a staging system and treatment strategies for HCC for the first time [4,5], evidence-based or consensus-based guidelines began to be developed in South Korea [6], Japan [7,8], China [9], and other countries. Each country had developed similar but different guidelines. Several factors contributed to these differences, such as the primary cause of HCC, prevalence, and characteristics of underlying diseases, varying staging systems, government and medical insurance reimbursement policies, medical resources, compliance of doctors and patients, and cultural differences. The guidelines developed in different countries differ fundamentally because there is a lack of concrete medical evidence, and even existing results of clinical trials can be interpreted differently. Fortunately, after 20 years of development and revision, various guidelines tend to converge similarly while influencing each other.
In this review, we summarized and compared the current guidelines of HCC in Asian countries including surveillance strategies, diagnostic modalities, staging systems, and treatment modalities, as well as locoregional and surgical treatment modalities. Additionally, we discussed the future management of HCC in Asia
SURVEILLANCE
Clinical practice guideline overview
Most Asia HCC practice guidelines recommend regular surveillance at six-month intervals for HCC in high-risk groups, including patients with chronic hepatitis B or C, and liver cirrhosis at a 6-onth interval. In China [10], the aMAP (age-Male-ALBI-Platelets) score was newly added in the 2022 Chinese guideline to help discriminate high-risk groups. Taiwanese guidelines recommend the HCC surveillance interval with a range of 6–12 months [11]. In Japan, liver ultrasonography (US) plus alpha-fetoprotein (AFP), des-gamma-carboxy prothrombin (DCP) and AFP-L3 testing are recommended every six months for the high-risk group (HBV/HCV infection, cirrhosis of other etiologies) and every three to four months for the extremely high-risk group (HBV/HCV-related cirrhosis) [12].
All guidelines recommend the combined use of serum AFP and liver US except the Asian Pacific Association for the Study of the Liver (APASL) guideline [13]. APASL guidelines suggest that AFP is not recommended as a confirmatory test in small HCCs, and the cut-off value of AFP should be set at 200 ng/mL for the surveillance program. APASL and Taiwanese guidelines recommend imaging modalities including contrast-enhanced ultrasonography (CEUS). Korean [14], Hong Kong [15], and Taiwanese [11] guidelines recommend dynamic contrast-enhanced computed tomography (CT) or dynamic contrast-enhanced magnetic resonance imaging (MRI) as an alternative to suboptimal US assessment.
Real-life practice
Many efforts and strategies are improving the adherence rate to HCC surveillance recommendations in Asia. The Korean government has initiated the National Liver Cancer Screening Program since 2003 [16]. The Bureau of National Health Insurance in Taiwan [17] started a medical care improvement plan for patients with chronic HBV and HCV infection since 2000. In China, lack of government-supported programs to cover surveillance for more population also contributed to a low diagnostic rate of early HCC [18].
Timely diagnosing of early-stage HCC and curative treatment are still suboptimal in Asia. According to the Korean National Health Insurance Service database, only 52.7% of high-risk individuals participated in the national liver cancer surveillance program during 2003–2015. More efforts to increase the adherence rate to surveillance should be performed in Asia. Also, alternative screening tools, including abbreviated MRI [19] should be validated as screening methods for HCC to increase sensitivity.
DIAGNOSIS
Clinical practice guideline overview
Korean [14], Japananese [12], and Taiwanese [11] guidelines recommend the diagnosis of HCC according to pathology or using typical radiologic hallmarks of HCC through dynamic contrast-enhanced CT or MRI, for a ≥1 cm nodule detected by surveillance among high-risk patients. In China [15], the diagnosis of HCC can be made if nodules >2 cm among high-risk patients with typical features on any of the three imaging modalities including multiphasic dynamic CT, MRI or Gadolinum (Gd)-EOB-DTPA-enhanced MRI, and CEUS. The diagnosis of nodules ≤2 cm in China can be established with typical imaging features on at least two modalities. APASL guideline also suggest that CEUS is useful and is as sensitive as dynamic CT or dynamic MRI in diagnosis of HCC. Korea [14] and APASL guidelines [13] highlight the importance of a combined interpretation of the dynamic and hepatobiliary phases of the Gd-EOB-DTPA-enhanced MRI with diffusion-weighted imaging [20].
Real-life practice
In Korea, radiological hallmarks include arterial phase hyperenhancement with washout appearance in multiphasic CT or MRI with extracellular contrast agent or MRI with liver-specific contrast [21] and CEUS [22]. A diagnosis of “probable” HCC can be made by applying ancillary imaging features. APASL guidelines [13] are frequently referenced by clinicians in Hong Kong. In Taiwan [11], physicians have adopted a more aggressive attitude toward active tumor biopsy, due to the booming numbers of immunotherapy combination trials in HCC with 48.2% of HCC diagnoses supported by pathology or cytology in 2019. In China [23], diagnostic accuracy is given priority over sensitivity due to the unbalanced distribution of medical resources. Therefore, confirmation of HCC diagnosis with two imaging modalities for nodules of 1–2 cm was adopted in China.
STAGING
Clinical practice guideline overview
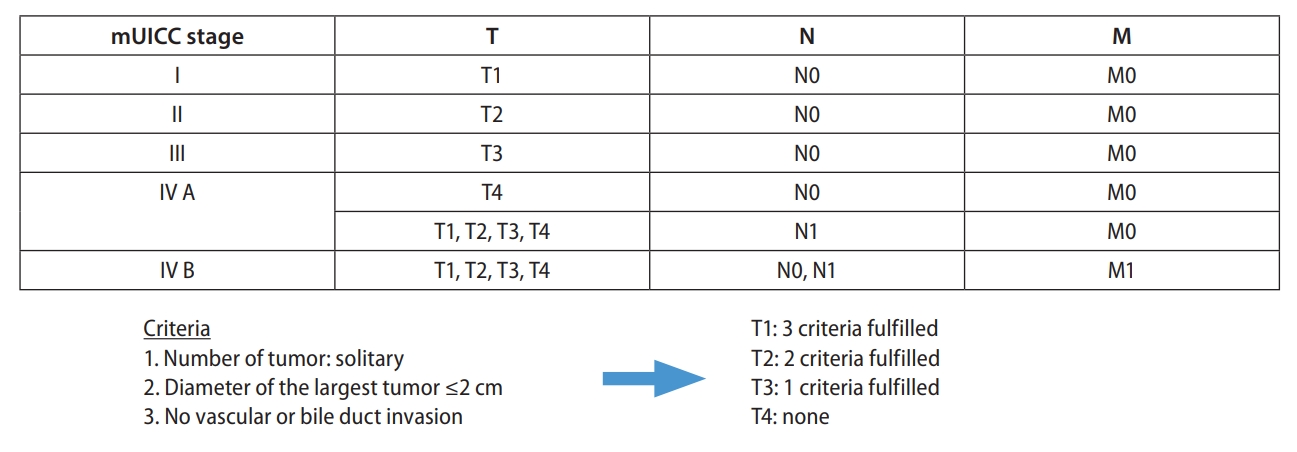
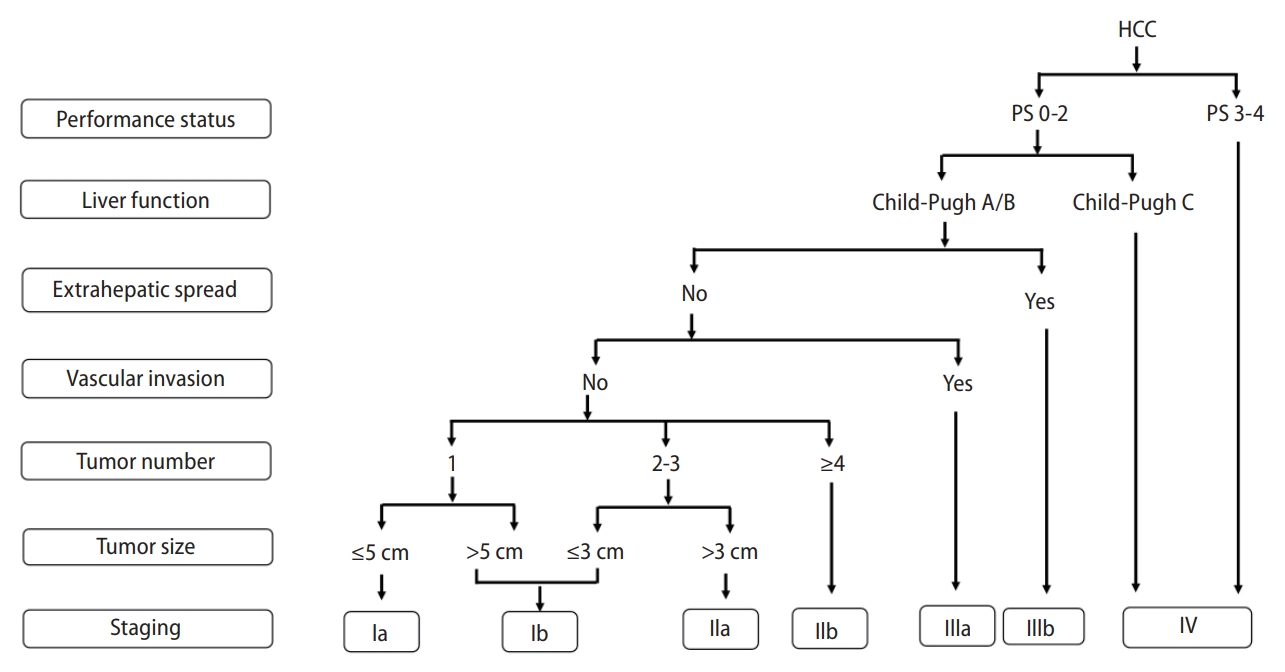
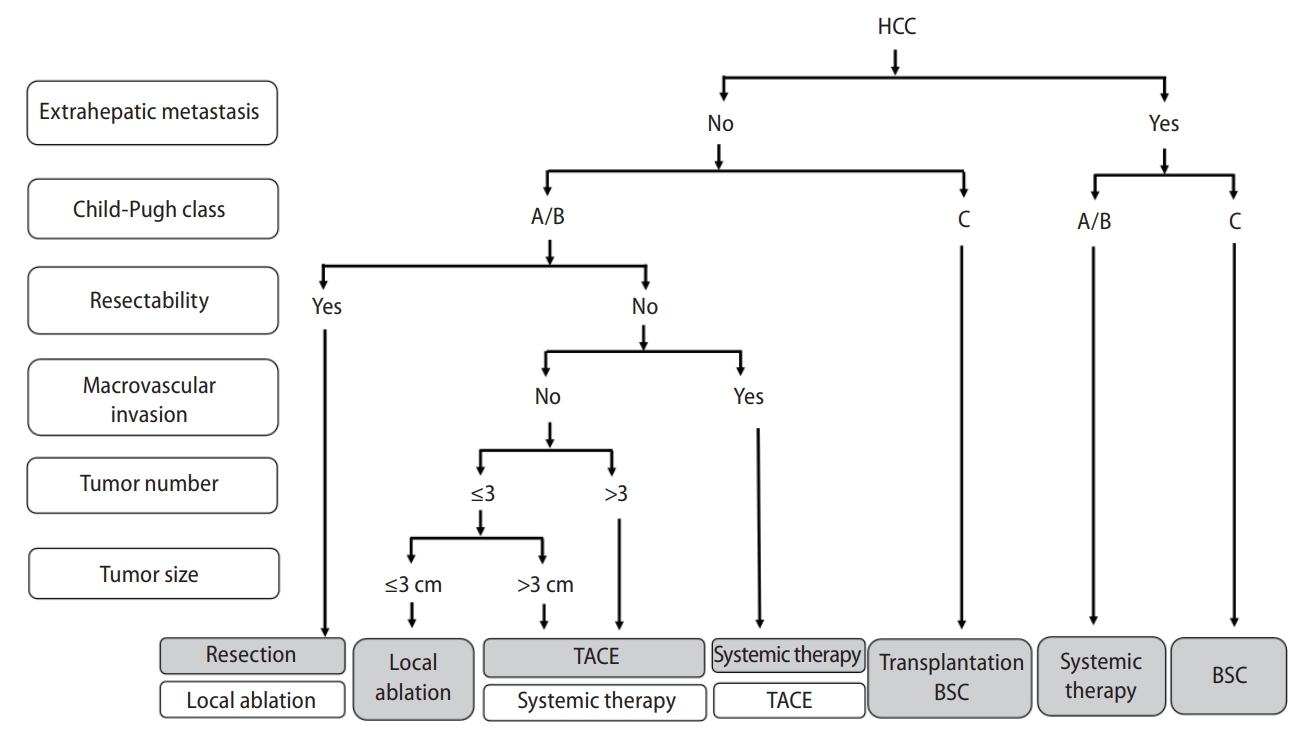
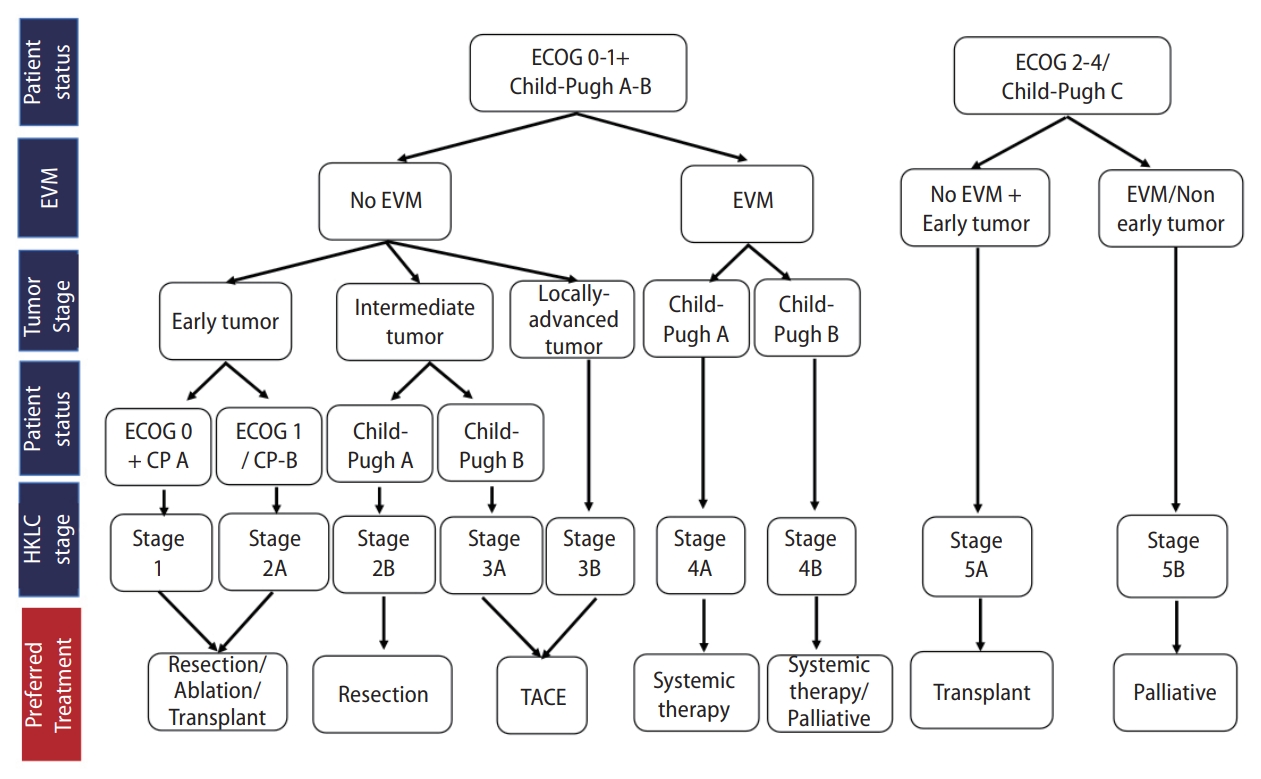
Korea [14] and Japan [12] guidelines adopted the modified Union for International Cancer Control (mUICC) stages as the primary staging system (Fig. 1). In Taiwan, the Barcelona Clinic of Liver Cancer (BCLC) staging system [24] is the most commonly used staging system in terms of prognostic prediction. Hong Kong consensus guidelines [15] suggest the Hong Kong Liver Cancer (HKLC) staging system (Fig. 2) as the staging system of choice, which was developed in 2014 with data from 3,856 HCC patients in Hong Kong. HKLC staging includes the Eastern Cooperative Oncology Group status, Child-Pugh score, the presence of extravascular invasion or metastasis, tumor size, and number of tumor nodules. In China, the China liver cancer staging system (Fig. 3) incorporating tumor characteristics, liver function, and performance status, which is similar to the BCLC system, was established in 2017. APASL guideline [13] also suggest a treatment algorithm (Fig. 4) according to extrahepatic metastasis, Child-Pugh class, resectability, macrovascular invasion, tumor number, or size.
Staging and preferred treatment in the Hong Kong Liver Cancer Staging (HKLC) system. EVM, extravascular metastasis; ECOG, Eastern Cooperative Oncology Group; TACE, transarterial chemoembolization.
Real-life practice
Korea guidelines [14] adopt mUICC staging as the primary system, however, the BCLC staging system and American Joint Committee on Cancer (AJCC)/UICC TNM staging system) also serve as complementary systems. Although the BCLC staging system is the most used staging system in Taiwan, the treatment algorithm suggested by BCLC staging system does not reflect the true daily practice in Taiwan and most Asian countries where diverse locoregional therapy and systemic therapies are available.
TREATMENT
Resection
Clinical practice guideline overview
Most Asian guidelines recommend surgical resection for single HCC of any size, as in the European or American guidelines [10,12,14,15,17,25,26]. Most Asian guidelines also recommend surgical resection for multiple tumors if lesions are localized [10,14,15,17,27,28]. Chinese guidelines suggest that surgical resection can be considered if tumors with more than four lesions or vascular invasion are confined in the same segment or lobe [10,28]. Hong Kong guidelines recommend surgical resection for solitary or multifocal lesions confined to the liver [15,27]. Taiwanese guidelines suggest surgical resection for multiple tumors in one lobe of the liver [17]. Even if tumors invade portal vein, surgical resection may be considered in selected patients according to Asian guidelines [10,12,15,17]. In other Asian countries except Korea, it is thought that the guidelines were developed mainly by surgeons based on consensus, and accordingly, the indications for surgery seem to have become wider.
For patients with inadequate future liver remnants, Chinese and Hong Kong guidelines suggest portal vein embolization or Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy (ALPPS), whereas American guidelines suggest portal vein embolization or transarterial radioembolization (TARE) to increase the chance of resection [10,15,26].
Real-life practice
Most Asian guidelines advocate surgical resection in more expanded indications [10,14,15,17,27-30]. Even in HCC patients with portal vein invasion, surgical resection has been performed in China, Hong Kong, and Taiwan [27,28,30].
Since surgical resection is more aggressively performed, several methods to improve resectability, such as portal vein embolization or ALPPS, were mentioned. However, ALPPS is limited and is only performed in specialized centers in Hong Kong [27]. Other than these methods, multi-modal treatment strategies such as systemic therapy, hepatic arterial infusion chemotherapy, or TACE have been performed to improve resectability [28].
Liver transplantation
Clinical practice guideline overview
Most Asian guidelines recommend liver transplantation as the primary treatment in patients with poor liver function [10,14,15,17,25,26,29]. Patient selection criteria differ from country to country. Taiwanese, Japan and Korean guidelines adopt the Milan criteria as the major criteria, while Chinese and Hong Kong guidelines adopt the University of California San Francisco (UCSF) criteria [10,12,14,15,17,25,26,29]. Asian countries are confronting a deceased donor shortage, and it is a critical barrier to liver transplantation. Most Asian guidelines mentioned living donor liver transplantation [10,12,14,15].
Real-life practice
Liver transplantation is an ideal treatment for patients with HCC and liver cirrhosis. However, organ shortage often precludes liver transplantation, and most Asian guidelines stated living donor liver transplantation [10,12,14,15]. Organ shortage in Taiwan leads to a salvage transplantation strategy; surgical resection is performed as the primary treatment, followed by transplantation in the case of recurrence of liver failure [30]. In Hong Kong, Japan and Korea, living donor liver transplantation is the predominant type of transplant surgery [12,14,27]. Sometimes, more expanded criteria beyond the Milan or UCSF criteria, or biomarker-based criteria can be applied for living donor liver transplantation [12,14,28].
Ablation
Clinical practice guideline overview
Most Asian guidelines recommend local ablation for very early- or early-stage HCCs ≤3 cm [10,12,14,15,17]. Chinese guidelines also recommended local ablation for solitary HCC ≤5 cm as a curative treatment [10]. Japanese guidelines recommend radiofrequency ablation for HCC with ≤3 cm and ≤3 nodules, and surgical resection for solitary HCC >3 cm as a primary choice [12,31]. Hong Kong and Taiwanese guidelines recommend local ablation in unsuitable cases for resection [15,17]. Microwave ablation as well as radiofrequency ablation are recommended as local ablation modalities.
Real-life practice
Local ablation therapies show similar overall survival and slightly higher local tumor progression, but lower complication rates compared with surgical resection. Larger tumor size or high-risk locations may lead to higher local tumor progression rates. Artificial ascites or pleural effusion infusion may be considered to overcome these limitations [10,12,17,30]. In China, local ablation in combination with TACE is considered for larger tumors [10,28].
Transarterial therapy
Clinical practice guideline overview
TACE is a widely used therapeutic modality for intermediate-stage HCC worldwide. However, each country’s staging method is slightly different; therefore, each country’s indications seem different from the intermediate stage of BCLC. Transarterial therapy using new materials such as drug-eluting beads (DEB), or radioisotope (yttrium-90) has been introduced. DEB-TACE and TARE offer a low incidence of postembolization syndrome, one of the painful adverse events of conventional TACE. Most Asian guidelines recommend TACE as a major treatment modality for intermediate-stage HCC without major vascular invasion or extrahepatic spread [10,12,14,15,17]. Hong Kong guidelines suggest TACE in HCC with portal vein invasion either alone (segmental portal vein invasion) or in combination with radiotherapy [15,27]. Taiwanese guidelines suggest that TACE can be considered in HCC with portal vein invasion in combination with targeted therapy, radiotherapy, TARE, or hepatic arterial infusion chemotherapy [17,30]. Japanese guidelines recommend TACE for HCCs with 2–3 tumors of ≥3 cm in diameter or ≥4 tumors primarily [12,31]. They also suggest conventional TACE for small tumors and DEB-TACE for large tumors since conventional TACE is theoretically more effective than DEB-TACE for small HCCs and DEB-TACE shows milder adverse events [12.31]. Korean guidelines also recommend drug-eluting bead TACE as an alternative treatment to conventional TACE in HCCs ≥3 cm since the local tumor response of DEB-TACE was significantly lower than that of conventional TACE in HCCs <3 cm [14,32], and TARE because of offering a better quality of life and lower incidence of postembolization syndrome [14].
Real-life practice
In real clinical practice for many Asian countries, TACE has been widely adopted as a primary option for early, intermediate, and advanced-stage HCC. Implementation of newer methods such as DEB-TACE and TARE depends on the policy of each government and the economic burden. In Taiwan, DEB-TACE and TARE are not reimbursed by the NHI program yet [30]. TARE is also less frequently performed in China and Hong Kong [27,28]. The costs of both DEB-TACE and TARE are still high since they are reimbursed partly by the government in Korea [14]; however, the use of TARE is increasing.
Systemic therapy
Clinical practice guideline overview
Most guidelines commonly recommend atezolizumab plus bevacizumab, sorafenib, and lenvatinib as a first-line option (Table 1) [10,11,14,15,29,31,33]. Hong Kong guidelines also recommended nivolumab for patients who have a contraindication to antiangiogenic therapies or tyrosine kinase inhibitors (TKIs) as first-line treatment [15]. Korean guidelines included tremelimumab plus durvalumab since the guidelines were most recently released (2022) [14]. Chinese guidelines adopted many systemic agents developed in China: donafenib and sintilimab plus bevacizumab biosimilar (Byvasda), apatinib, camrelizumab, and tislelizumab [29]. As first-line treatment, Chinese guidelines recommended atezolizumab plus bevacizumab, lenvatinib, sorafenib, donafenib, sintilimab plus bevacizumab biosimilar (Byvasda), and FOLFOX [10,29].
As a second-line option, regorafenib, cabozantinib, ramucirumab, nivolumab with or without ipilimumab, and pembrolizumab were recommended in Hong Kong, Taiwan, and Korea [11,14,15]. Chinese guidelines recommended regorafenib, apatinib, camrelizumab, and tislelizumab as second-line treatment [10,29].
Japanese guidelines recommend regorafenib, cabozantinib, and ramucirumab as a second-line option. Japanese guidelines also recommend sorafenib or lenvatinib for patients who did not experience sorafenib or lenvatinib as a first-line option, respectively [31].
Real-life practice
A couple of new systemic agents have been introduced recently. Major study results as well as the approval and/or reimbursement status incorporate into the real clinical practice.
First-line immunotherapy is not reimbursed yet in Taiwan; therefore, atezolizumab plus bevacizumab or lenvatinib plus pembrolizumab were given based on shared decision-making [30]. In Hong Kong, nivolumab plus ipilimumab is also given as a first-line therapy [27]. In China, PD-1/PD-L1 inhibitors plus TKIs have been in wide use in real clinical practice [28]. In Korea [14], only one option among atezolizumab plus bevacizumab, lenvatinib, and sorafenib is reimbursed as a first-line option; atezolizumab plus bevacizumab is most commonly chosen nowadays.
Most second-line treatments are recommended for sorafenib failure. Recommendations on subsequent therapy after atezolizumab plus bevacizumab or lenvatinib is based on experts’ opinion [14,27,28,30]. In Hong Kong guidelines, regorafenib, cabozantinib, ramucirumab or unused TKI, and immunotherapy could be considered after lenvatinib failure [15,27]. Nivolumab with or without ipilimumab and pembrolizumab can be considered after approved TKIs (lenvatinib, sorafenib) in Taiwan [30]. In Japan, sorafenib or lenvatinib is recommended after atezolizumab plus bevacizumab failure [12,31]. Korean guidelines [14] mentioned that unused TKI or IO drugs that do not share mechanisms of action can be given to lenvatinib or first-line IO-based doublet therapy failures; however, all subsequent agents are not reimbursed as second-line treatment after lenvatinib or atezolizumab plus bevacizumab as of March 2022.
External beam radiation therapy
Clinical practice guideline overview
Most Asian guidelines recommend external beam radiation therapy in selected patients, while Western guidelines suggest that external beam radiotherapy is under investigation since there is a lack of robust evidence. Radiotherapy can be given as an alternative to surgical resection or locoregional therapeutic modalities (local ablation or TACE) or as a palliative modality. In China, stereotactic body radiation therapy (SBRT) is indicated for patients with solitary lesions or multiple tumors ≤3 cm who are ineligible for curative treatment [10]. Radiotherapy can be given to patients with multiple HCCs in combination with TACE or tumor thrombus or oligometastasis [10,28]. Hong Kong guidelines suggest SBRT as an alternative to other liver-directed therapies and SBRT can also be given in combination with TACE for large tumors (>5 cm) [15]. Taiwanese guidelines suggest radiotherapy for HCCs ineligible or inaccessible to local ablation or TACE, or HCCs with portal vein invasion [17,30]. Radiotherapy may also be considered for symptomatic metastasis or oligometastasis [15]. Korean guidelines recommend radiotherapy patients with HCC unsuitable for hepatic resection, transplantation, or locoregional therapy [14]. Radiotherapy can be additionally performed for HCC showing incomplete response to TACE or with portal vein invasion [14]. Korean guidelines also recommend proton beam radiotherapy since it showed comparable local tumor control and overall survival compared with radiofrequency ablation in HCCs ≤3 cm [14,34].
Real-life practice
Radiotherapy has been widely used in Asia as an alternative therapy to curative treatment or in combination with other locoregional therapies. Radiotherapy is versatile and the favored treatment modality [27,28,30]. SBRT can deliver ablative radiation dose in low fractions and SBRT for unresectable HCC either alone or in combination with TACE is increasing in Hong Kong [27]. Also in Korea, radiotherapy is used for HCCs unsuitable for surgical resection, local ablation, or TACE [14,35]. Radiotherapy may also be performed in combination with TACE or systemic therapy [14,35].
DISCUSSION
HCC is a heterogeneous and complex disease in which multidisciplinary approaches are necessary to optimize management. In contrast to other cancers, the regional differences in etiology-dependent tumor biology and socio-medical resources make it impractical to have a globally universal guideline for all patients with HCC. Asian HCC practice guidelines are similar but different from each other because of similar main causes (i.e., HBV infection) but different interpretations of results from clinical trials secondary to a lack of concrete, high-level evidence. Therefore, it is serious to acknowledge these challenges and devise effective strategies to overcome them, enhance the design of upcoming clinical trials, and finally, improve the outcomes of HCC patients worldwide. Since the liver is an immune-tolerant organ [36,37]. it was assumed that immune checkpoint inhibitor therapy of HCC would be disadvantageous; however, recently, atezolizumab plus bevacizumab [38] and durvalumab plus tremelimumab [39] have succeeded in clinical trials, making them available for advanced patients in some countries, and recommended in the most consistently universal guidelines. Like such achievements, multidisciplinary efforts to optimize treatment and improve clinical trial designs are ultimately necessary.
Despite some mutual criticism and rejection, Eastern and Western physicians have learned from each other regarding improving patient outcomes. A better understanding of differences in oncological background and socio-economic resources in different countries can help make better multinational clinical trials and provide better outcomes, from which more concrete evidence and an indisputable recommendation could be developed in most countries. Because consensus-based recommendations are more influenced by socioeconomic policies, medical resources, leading physicians, compliance of patients, and culture, it is critical to remember those influences when understanding the recommendations.
Improving the survival rates of patients with HCC across all stages is a crucial goal that can be achieved by developing more effective biomarkers for prediction and personalized therapeutic approaches based on well-designed multinational clinical trials.
Notes
Authors’ contribution
Study conceptualization: JWP; Drafting of the manuscript: YC, BHK ; Critical revision of the manuscript: YC, BHK, and JWP.
Conflicts of Interest
The authors have no conflicts to disclose.
Abbreviations
AFP
alpha-fetoprotein
ALPPS
Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy
aMAP
age-Male-ALBI-Platelets score
APASL
Asian Pacific Association for the Study of the Liver
CEUS
contrast-enhanced ultrasonography
CHB
chronic hepatitis B
CT
computed tomography
DCP
des-gamma-carboxy prothrombin
DEB
drug-eluting beads
Gd
Gadolinum
HBV
hepatitis B virus
HCC
hepatocellular carcinoma
HCV
hepatitis C virus
IO
immune-oncology
MRI
magnetic resonance imaging
SBRT
stereotactic body radiation therapy
TACE
transarterial chemoembolization
TARE
transarterial radioembolization
TKI
tyrosine kinase inhibitor
UCSF
University of California San Francisco
US
ultrasonography