3. Kao JH, Chen PJ, Chen DS. Recent advances in the research of hepatitis B virus-related hepatocellular carcinoma: epidemiologic and molecular biological aspects. Adv Cancer Res 2010;108:21-72.

6. Lin CL, Kao JH. Hepatitis B: Immunization and impact on natural history and cancer incidence. Gastroenterol Clin North Am 2020;49:201-214.

8. Chen CL, Yang JY, Lin SF, Sun CA, Bai CH, You SL, et al. Slow decline of hepatitis B burden in general population: Results from a population-based survey and longitudinal follow-up study in Taiwan. J Hepatol 2015;63:354-363.


9. Kao JH, Chen DS. Global control of hepatitis B virus infection. Lancet Infect Dis 2002;2:395-403.


15. Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, et al.; REVEAL-HBV Study Group. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006;295:65-73.


16. Liu CJ, Chen BF, Chen PJ, Lai MY, Huang WL, Kao JH, et al. Role of hepatitis B viral load and basal core promoter mutation in hepatocellular carcinoma in hepatitis B carriers. J Infect Dis 2006;193:1258-1265.


17. Liu CJ, Chen BF, Chen PJ, Lai MY, Huang WL, Kao JH, et al. Role of hepatitis B virus precore/core promoter mutations and serum viral load on noncirrhotic hepatocellular carcinoma: a case-control study. J Infect Dis 2006;194:594-599.


18. Yuen MF, Tanaka Y, Fong DY, Fung J, Wong DK, Yuen JC, et al. Independent risk factors and predictive score for the development of hepatocellular carcinoma in chronic hepatitis B. J Hepatol 2009;50:80-88.


19. Wong VW, Chan SL, Mo F, Chan TC, Loong HH, Wong GL, et al. Clinical scoring system to predict hepatocellular carcinoma in chronic hepatitis B carriers. J Clin Oncol 2010;28:1660-1665.


22. Zoulim F, Mason WS. Reasons to consider earlier treatment of chronic HBV infections. Gut 2012;61:333-336.


25. Xu C, Yamamoto T, Zhou T, Aldrich CE, Frank K, Cullen JM, et al. The liver of woodchucks chronically infected with the woodchuck hepatitis virus contains foci of virus core antigennegative hepatocytes with both altered and normal morphology. Virology 2007;359:283-294.


28. Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol 2016;64(1 Suppl):S84-S101.


32. P├®neau C, Imbeaud S, La Bella T, Hirsch TZ, Caruso S, Calderaro J, et al. Hepatitis B virus integrations promote local and distant oncogenic driver alterations in hepatocellular carcinoma. Gut 2022;71:616-626.


34. Fako V, Wang XW. Molecular carcinogenesis of HBV-related HCC. In: Kao JH, Chen DS, eds. Hepatitis B Virus and Liver Disease. Singapore: Springer Nature Singapore Pte Ltd.; 2018. p. 143-162.
36. Tseng TC, Liu CJ, Yang HC, Chen CL, Yang WT, Tsai CS, et al. Higher proportion of viral basal core promoter mutant increases the risk of liver cirrhosis in hepatitis B carriers. Gut 2015;64:292-302.


42. Global Burden of Disease Liver Cancer Collaboration, Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: Results from the global burden of disease study 2015. JAMA Oncol 2017;3:1683-1691.


45. Yu MW, Lin CL, Liu CJ, Yang SH, Tseng YL, Wu CF. Influence of metabolic risk factors on risk of hepatocellular carcinoma and liver-related death in men with chronic hepatitis B: A large cohort study. Gastroenterology 2017;153:1006-1017.e5.


48. Zucman-Rossi J, Villanueva A, Nault JC, Llovet JM. Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology 2015;149:1226-1239.e4.


51. Huang DQ, Li X, Le MH, Le AK, Yeo YH, Trinh HN, et al. Natural history and hepatocellular carcinoma risk in untreated chronic hepatitis B patients with indeterminate phase. Clin Gastroenterol Hepatol 2022;20:1803-1812.e5.


52. Chen JD, Yang HI, Iloeje UH, You SL, Lu SN, Wang LY, et al.; Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer in HBV (REVEAL-HBV) Study Group. Carriers of inactive hepatitis B virus are still at risk for hepatocellular carcinoma and liver-related death. Gastroenterology 2010;138:1747-1754.


53. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. Erratum in: CA Cancer J Clin 2021;71:359.
54. Sinn DH, Lee J, Goo J, Kim K, Gwak GY, Paik YH, et al. Hepatocellular carcinoma risk in chronic hepatitis B virus-infected compensated cirrhosis patients with low viral load. Hepatology 2015;62:694-701.


55. Tseng TC, Liu CJ, Yang HC, Su TH, Wang CC, Chen CL, et al. High levels of hepatitis B surface antigen increase risk of hepatocellular carcinoma in patients with low HBV load. Gastroenterology 2012;142:1140-1149.e3 quiz e13-14.


60. Tada T, Kumada T, Toyoda H, Kiriyama S, Tanikawa M, Hisanaga Y, et al. HBcrAg predicts hepatocellular carcinoma development: An analysis using time-dependent receiver operating characteristics. J Hepatol 2016;65:48-56.


61. Tseng TC, Liu CJ, Hsu CY, Hong CM, Su TH, Yang WT, et al. High level of hepatitis B core-related antigen associated with increased risk of hepatocellular carcinoma in patients with chronic HBV infection of intermediate viral load. Gastroenterology 2019;157:1518-1529.e3.


62. Tseng TC, Hosaka T, Liu CJ, Suzuki F, Hong CM, Kumada H, et al. Hepatitis B core-related antigen stratifies the risk of liver cancer in HBeAg-negative patients with indeterminate phase. Am J Gastroenterol 2022;117:748-757.


68. Lin CL, Liu CH, Wang CC, Liang CC, Su TH, Liu CJ, et al. Serum biomarkers predictive of significant fibrosis and cirrhosis in chronic hepatitis B. J Clin Gastroenterol 2015;49:705-713.


69. Tseng TC, Liu CJ, Su TH, Yang WT, Chen CL, Yang HC, et al. Fibrosis-4 index helps identify HBV carriers with the lowest risk of hepatocellular carcinoma. Am J Gastroenterol 2017;112:1564-1574.


74. Lichtinghagen R, Pietsch D, Bantel H, Manns MP, Brand K, Bahr MJ. The Enhanced Liver Fibrosis (ELF) score: normal values, influence factors and proposed cut-off values. J Hepatol 2013;59:236-242. Erratum in: J Hepatol 2013;59:1365.
75. Fernandes FF, Ferraz ML, Andrade LE, Dellavance A, Terra C, Pereira G, et al. Enhanced liver fibrosis panel as a predictor of liver fibrosis in chronic hepatitis C patients. J Clin Gastroenterol 2015;49:235-241.


77. Gupta S, Bent S, Kohlwes J. Test characteristics of alphafetoprotein for detecting hepatocellular carcinoma in patients with hepatitis C. A systematic review and critical analysis. Ann Intern Med 2003;139:46-50.


81. Yi X, Yu S, Bao Y. Alpha-fetoprotein-L3 in hepatocellular carcinoma: a meta-analysis. Clin Chim Acta 2013;425:212-220.


86. Yang HI, Yuen MF, Chan HL, Han KH, Chen PJ, Kim DY, et al.; REACH-B Working Group. Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): development and validation of a predictive score. Lancet Oncol 2011;12:568-574.


87. Lee MH, Yang HI, Liu J, Batrla-Utermann R, Jen CL, Iloeje UH, et al.; R.E.V.E.A.L.-HBV Study Group. Prediction models of long-term cirrhosis and hepatocellular carcinoma risk in chronic hepatitis B patients: risk scores integrating host and virus profiles. Hepatology 2013;58:546-554.


88. Yang HI, Tseng TC, Liu J, Lee MH, Liu CJ, Su TH, et al. Incorporating serum level of hepatitis B surface antigen or omitting level of hepatitis B virus DNA does not affect calculation of risk for hepatocellular carcinoma in patients without cirrhosis. Clin Gastroenterol Hepatol 2016;14:461-468.e2.


89. Wong GL, Chan HL, Wong CK, Leung C, Chan CY, Ho PP, et al. Liver stiffness-based optimization of hepatocellular carcinoma risk score in patients with chronic hepatitis B. J Hepatol 2014;60:339-345.


90. Poh Z, Shen L, Yang HI, Seto WK, Wong VW, Lin CY, et al. Realworld risk score for hepatocellular carcinoma (RWS-HCC): a clinically practical risk predictor for HCC in chronic hepatitis B. Gut 2016;65:887-888.


92. Fan C, Li M, Gan Y, Chen T, Sun Y, Lu J, et al. A simple AGED score for risk classification of primary liver cancer: development and validation with long-term prospective HBsAg-positive cohorts in Qidong, China. Gut 2019;68:948-949.


94. Abu-Amara M, Cerocchi O, Malhi G, Sharma S, Yim C, Shah H, et al. The applicability of hepatocellular carcinoma risk prediction scores in a North American patient population with chronic hepatitis B infection. Gut 2016;65:1347-1358.


96. European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370-398.


98. Sung JJ, Tsoi KK, Wong VW, Li KC, Chan HL. Meta-analysis: Treatment of hepatitis B infection reduces risk of hepatocellular carcinoma. Aliment Pharmacol Ther 2008;28:1067-1077.


99. Yang YF, Zhao W, Zhong YD, Xia HM, Shen L, Zhang N. Interferon therapy in chronic hepatitis B reduces progression to cirrhosis and hepatocellular carcinoma: a meta-analysis. J Viral Hepat 2009;16:265-271.


100. Wong GL, Yiu KK, Wong VW, Tsoi KK, Chan HL. Meta-analysis: reduction in hepatic events following interferon-alfa therapy of chronic hepatitis B. Aliment Pharmacol Ther 2010;32:1059-1068.


101. Liang KH, Hsu CW, Chang ML, Chen YC, Lai MW, Yeh CT. Peginterferon is superior to nucleos(t)ide analogues for prevention of hepatocellular carcinoma in chronic hepatitis B. J Infect Dis 2016;213:966-974.


102. Ren P, Cao Z, Mo R, Liu Y, Chen L, Li Z, et al. Interferon-based treatment is superior to nucleos(t)ide analog in reducing HBV-related hepatocellular carcinoma for chronic hepatitis B patients at high risk. Expert Opin Biol Ther 2018;18:1085-1094.


104. Nguyen MH, Yang HI, Le A, Henry L, Nguyen N, Lee MH, et al. Reduced incidence of hepatocellular carcinoma in cirrhotic and noncirrhotic patients with chronic hepatitis B treated with tenofovir-A propensity score-matched study. J Infect Dis 2019;219:10-18.


107. Tseng CH, Hsu YC, Chen TH, Ji F, Chen IS, Tsai YN, et al. Hepatocellular carcinoma incidence with tenofovir versus entecavir in chronic hepatitis B: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2020;5:1039-1052. Erratum in: Lancet Gastroenterol Hepatol 2021;6:e1.
112. Testoni B, Leboss├® F, Scholtes C, Berby F, Miaglia C, Subic M, et al. Serum hepatitis B core-related antigen (HBcrAg) correlates with covalently closed circular DNA transcriptional activity in chronic hepatitis B patients. J Hepatol 2019;70:615-625.


115. Hosaka T, Suzuki F, Kobayashi M, Fujiyama S, Kawamura Y, Sezaki H, et al. Impact of hepatitis B core-related antigen on the incidence of hepatocellular carcinoma in patients treated with nucleos(t)ide analogues. Aliment Pharmacol Ther 2019;49:457-471. Erratum in: Aliment Pharmacol Ther 2019;50:234-235.
122. Papatheodoridis G, Dalekos G, Sypsa V, Yurdaydin C, Buti M, Goulis J, et al. PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J Hepatol 2016;64:800-806.


123. Kim JH, Kim YD, Lee M, Jun BG, Kim TS, Suk KT, et al. Modified PAGE-B score predicts the risk of hepatocellular carcinoma in Asians with chronic hepatitis B on antiviral therapy. J Hepatol 2018;69:1066-1073.


126. Hsu YC, Yip TC, Ho HJ, Wong VW, Huang YT, El-Serag HB, et al. Development of a scoring system to predict hepatocellular carcinoma in Asians on antivirals for chronic hepatitis B. J Hepatol 2018;69:278-285. Erratum in: J Hepatol 2019;70:581.
127. Yu JH, Suh YJ, Jin YJ, Heo NY, Jang JW, You CR, et al. Prediction model for hepatocellular carcinoma risk in treatment-naive chronic hepatitis B patients receiving entecavir/tenofovir. Eur J Gastroenterol Hepatol 2019;31:865-872.


131. Dienstag JL, Goldin RD, Heathcote EJ, Hann HW, Woessner M, Stephenson SL, et al. Histological outcome during long-term lamivudine therapy. Gastroenterology 2003;124:105-117.


132. Schiff ER, Lee SS, Chao YC, Kew Yoon S, Bessone F, Wu SS, et al. Long-term treatment with entecavir induces reversal of advanced fibrosis or cirrhosis in patients with chronic hepatitis B. Clin Gastroenterol Hepatol 2011;9:274-276.


133. Chang TT, Liaw YF, Wu SS, Schiff E, Han KH, Lai CL, et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology 2010;52:886-893.


134. Chon YE, Park JY, Myoung SM, Jung KS, Kim BK, Kim SU, et al. Improvement of liver fibrosis after long-term antiviral therapy assessed by fibroscan in chronic hepatitis B patients with advanced fibrosis. Am J Gastroenterol 2017;112:882-891. Erratum in: Am J Gastroenterol 2017;112:1625.
137. Chon HY, Lee HA, Suh SJ, Lee JI, Kim BS, Kim IH, et al.; Korean Transient Elastography Study Group. Addition of liver stiffness enhances the predictive accuracy of the PAGE-B model for hepatitis B-related hepatocellular carcinoma. Aliment Pharmacol Ther 2021;53:919-927. Erratum in: Aliment Pharmacol Ther 2021;53:959-960.
138. Kao JH. Hepatitis B vaccination and prevention of hepatocellular carcinoma. Best Pract Res Clin Gastroenterol 2015;29:907-917.


139. Hung GY, Horng JL, Yen HJ, Lee CY, Lin LY. Changing incidence patterns of hepatocellular carcinoma among age groups in Taiwan. J Hepatol 2015;63:1390-1396.


140. Hsu YC, Chen CY, Chang IW, Chang CY, Wu CY, Lee TY, et al. Once-daily tenofovir disoproxil fumarate in treatment-naive Taiwanese patients with chronic hepatitis B and minimally raised alanine aminotransferase (TORCH-B): a multicentre, double-blind, placebo-controlled, parallel-group, randomised trial. Lancet Infect Dis 2021;21:823-833. Erratum in: Lancet Infect Dis 2021;21:e122.
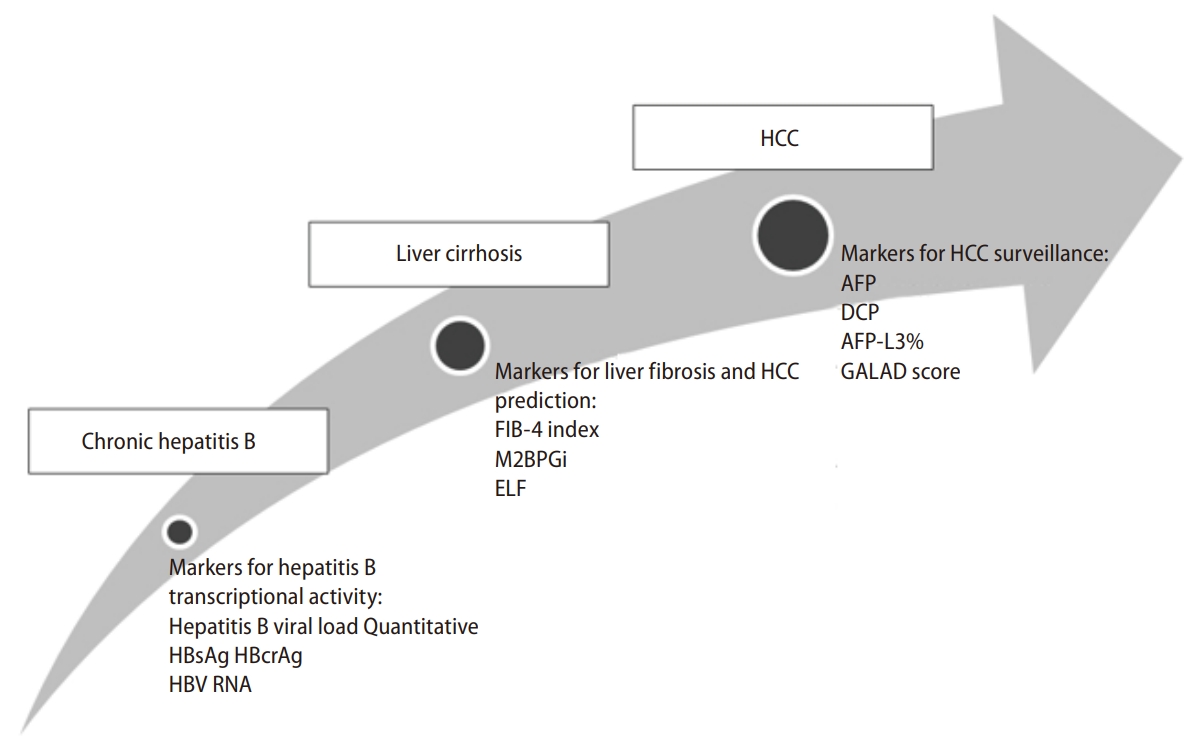
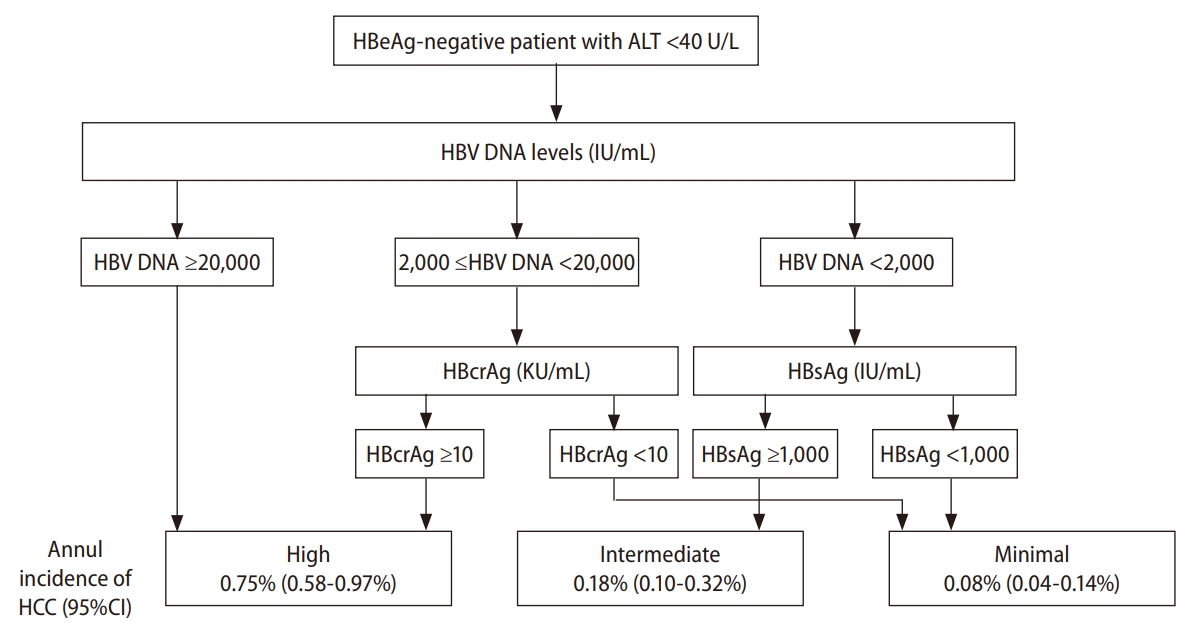
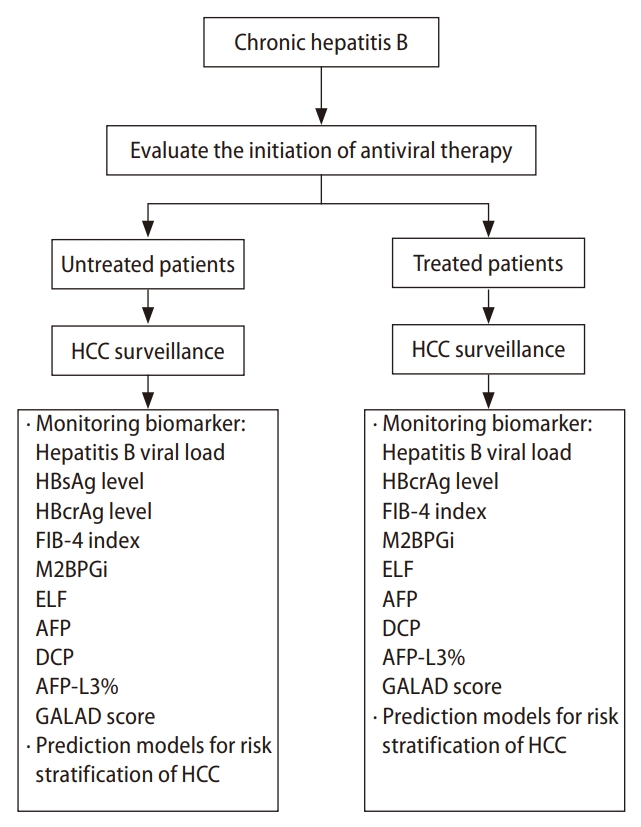





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



