Pathogenesis and treatment of non-alcoholic steatohepatitis and its fibrosis
Article information
Abstract
The initial presentation of non-alcoholic steatohepatitis (NASH) is hepatic steatosis. The dysfunction of lipid metabolism within hepatocytes caused by genetic factors, diet, and insulin resistance causes lipid accumulation. Lipotoxicity, oxidative stress, mitochondrial dysfunction, and endoplasmic reticulum stress would further contribute to hepatocyte injury and death, leading to inflammation and immune dysfunction in the liver. During the healing process, the accumulation of an excessive amount of fibrosis might occur while healing. During the development of NASH and liver fibrosis, the gut-liver axis, adipose-liver axis, and renin-angiotensin system (RAS) may be dysregulated and impaired. Translocation of bacteria or its end-products entering the liver could activate hepatocytes, Kupffer cells, and hepatic stellate cells, exacerbating hepatic steatosis, inflammation, and fibrosis. Bile acids regulate glucose and lipid metabolism through Farnesoid X receptors in the liver and intestine. Increased adipose tissue-derived non-esterified fatty acids would aggravate hepatic steatosis. Increased leptin also plays a role in hepatic fibrogenesis, and decreased adiponectin may contribute to hepatic insulin resistance. Moreover, dysregulation of peroxisome proliferator-activated receptors in the liver, adipose, and muscle tissues may impair lipid metabolism. In addition, the RAS may contribute to hepatic fatty acid metabolism, inflammation, and fibrosis. The treatment includes lifestyle modification, pharmacological therapy, and non-pharmacological therapy. Currently, weight reduction by lifestyle modification or surgery is the most effective therapy. However, vitamin E, pioglitazone, and obeticholic acid have also been suggested. In this review, we will introduce some new clinical trials and experimental therapies for the treatment of NASH and related fibrosis.
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is currently the most prevalent type of liver disease worldwide. NAFLD is a wide hepatic spectrum, ranging from simple steatosis to non-alcoholic steatohepatitis (NASH), which leads to progressive fibrosis, cirrhosis, and hepatocellular carcinoma (HCC) [1]. Fat accumulation in hepatocytes sensitizes hepatocytes to injury, leading to cell death, inflammatory cells recruitment, and activation of hepatic stellate cells (HSCs) [2]. The pathogenesis of NASH and its fibrosis has been broadly investigated for decades, and the development and progression of NASH and liver fibrosis involves complex interplay of numerous determinants. Understanding of the pathogenesis of NASH and liver fibrosis is important for the diagnosis and development of treatment. Although new drugs have been developed to target liver inflammation and fibrosis in NASH, only a minority of patients achieve treatment response [3]. Thus, there is still an urgent need to develop new therapeutic agents for NASH.
PATHOGENESIS OF NASH
Development of hepatic steatosis
Diet
High-fat diet can result in hepatic steatosis in humans. Liver fat increased by 35% in overweight non-diabetic women after a 2-week isocaloric high-fat diet (56% total energy from fat) [4]. A 3 days of high-fat, high-energy diet in healthy males resulted in major increases in plasma triglyceride (TG) and non-esterified fatty acid (NEFA) concentrations and hepatic TG [5]. A single energy-dense, high-fat meal induced net lipid accumulation in the liver of healthy subjects [6]. Moreover, palm oil administration in lean, healthy individuals decreased whole-body, hepatic, and adipose tissue insulin sensitivity by 25%, 15%, and 34%, respectively; increased hepatic TG and ATP content by 35% and 16%, respectively; increased hepatic gluconeogenesis by 70%; and decreased glycogenolysis by 20% [7]. In young Finnish adults, serum fatty acid saturation independently predicted the 10-year risk for fatty liver and omega-6 (ω6) fatty acids inversely associated with fatty liver [8]. A long-term hypercaloric diet, rich in saturated fatty acid (SFA), showed a marked increase in liver fat content by 50%, and ω6 polyunsaturated fatty acids (PUFAs) decreased fatty liver in overweight humans [9]. However, a lipidomic analysis showed that the n-6:n-3 free fatty acids (FFAs) ratio increased in NASH livers as compared to normal livers [10]. These studies suggested that hypercaloric diet, especially high in fat and sugar, contribute to the development of fatty liver; SFA and fructose are more detrimental, but the role of the ω6/ω3 fat ratio also remains controversial.
Physical inactivity
NAFLD patients have low level of physical activity compared to normal controls. Gerber et al. [11] showed that the average physical activity, counted by an accelerometer of NAFLD subjects, was about 28.7 counts/minute/day. In an Asian group, prolonged sitting time and decreased physical activity level were found to positively associated with the prevalence of NAFLD, and these associations were also observed in subjects with body mass index <23 kg/m2 [12]. However, the detail mechanism of sedentary behavior or low physical activity leading to fatty liver remains unclear. Lower expenditure of energy or lower skeletal muscle mass might explain a possible connection between sedentary behavior and NAFLD.
Insulin resistance
NAFLD is strongly associated with reduced whole body insulin sensitivity, as well as increased hepatic and adipose tissue insulin resistance [13,14]. Insulin resistance can lead to hepatic fat accumulation by increasing FFA delivery to the liver, increasing de novo lipogenesis (DNL), and decreasing hepatic fatty acid oxidation. A landmark study performed by Donnelly et al. [15] demonstrated that in NAFLD patients, about 59% liver triacylglycerol arose from NEFAs, 26.1% from DNL, and 14.9% from the diet, and that the liver demonstrated reciprocal use of adipose and dietary fatty acids. DNL was elevated in the fasting state without diurnal variation [15]. Insulin resistance can impair the insulin suppression of lipolysis of peripheral adipose tissues, leading to increased delivery of FFAs to the liver [16]. Insulin can stimulate sterol receptor binding protein 1-c (SREBP1c), increasing DNL in the liver [17,18]. Chronic hyperinsulinemia results in the cytoplasmic localization and inactivation of Foxa2 phosphorylation in hepatocytes, thereby promoting lipid accumulation and insulin resistance in the liver [19].
Genetic factors
There are several gene variants associated with NAFLD and NASH. The first fatty liver gene identified by Romeo et al. [20] is patatin-like phospholipase domain-containing 3 (PNPLA3). The single nucleotide polymorphism (SNP) rs738409 causes the missense sequence variation I148M, impairing the phospholipase activity and increasing hepatic fat content [20]. Glucokinase regulatory protein (GCKR) can regulate hepatic glucose uptake and hepatic glucokinase activity, and the intronic SNP rs780094 is associated with hepatic lipid content [21,22]. The SNP rs1260326 (C>T; P446L), GCKRP446L can decrease the inhibition of glucokinase, leading to increased glycolytic flux to hepatocytes, then hepatic steatosis [23]. The rs58542926 (G>A; E167K) variant, transmembrane 6 superfamily 2 (TM6SF2), was associated with increased hepatic TG content [24]. The inhibition of TM6SF2 in hepatocytes reduced the secretion of very-low-density lipoprotein (VLDL), leading to the retention of TGs [25]. In a Taiwanese population, a variant in the immunity-related GTPase M (IRGM) gene (rs10065172 TT genotype) independently increased the odds ratio of NAFLD by 2.04 by altering hepatic lipid metabolism through the autophagy pathway [26]. Similarly, the IRGM rs10065172 variant increased the risk for hepatic steatosis, but not for liver inflammation or fibrosis, in obese Italian children [27]. Recently, the mechanisms underlying metabolic and genetic components of NAFLD were found to be fundamentally different in patients. The metabolic component is characterized by hepatic oversupply of sugars and lipids, while the genetic component is characterized by impaired hepatic mitochondrial function, reducing the liver’s ability to metabolize these substrates [28].
Epigenetic factor
Using an epigenome-wide association study in peripheral blood cells, 22 CpGs were found to be associated with hepatic fat in European participants; 19 CpGs were annotated to 18 unique genes upregulated in the liver, including DHCR24, SLC43A1, CPT1A, SREBF1, SC4MOL, and SLC9A3R1 [29]. Some alternations of intrahepatic microRNA (miRNA) have been associated with hepatic steatosis. The serum levels of miR-122 and miR-192 were upregulated in patients with simple steatosis compared to normal controls [30]. The administration of exosomes transfected with obesity-associated miRNA induced hepatic steatosis in lean mice [31]. miR-122 inhibition in normal mice caused increased hepatic fatty acid oxidation [32]. Decreased miR-122-5p in the human liver was associated with impaired fatty acid usage [33]. However, the deletion of mouse miR-122 resulted in hepatosteatosis, inflammation, and the development of tumors [34]. The expression of miR-34 was elevated in NAFLD patients. miR-34a down-regulated autophagy in hepatocytes by targeting ATG4B and Rab-8B and suppressed mitochondrial biogenesis, leading to lipids accumulation in the liver [35].
Lipotoxicity
Endoplasmic reticulum (ER) stress
The ER is responsible for protein folding, and the accumulation of misfolded or unfolded proteins leads to stress and the activation of the unfolded protein response (UPR) [36]. There are three sensor proteins that activate UPR, namely the inositol-requiring enzyme 1 (IRE1), the protein kinase R (doublestranded RNA-activated protein kinase)-like ER kinases (PERK), and the activating transcription factor 6. The UPR can cause inflammation, inflammasome activation, and death of hepatocytes [37]. Patients with NASH have been shown to be specifically associated with failure to generate X-box-binding protein 1 (XBP-1) protein and activation of JNK [38]. Palmitate can induce the ER stress response, as demonstrated by the increase in C/EBP homologous protein (CHOP) expression, eIF2-alpha phosphorylation, XBP-1 splicing, and JNK activation with increased expression of the BH3-only proteins PUMA and Bim [39]. Perturbation of membrane lipid composition could promote IRE1 and PERK activation, suggesting a lipid-sensing mechanism for ER sensors to activate the UPR [40]. NFATc1 drives hepatocyte damage and inflammation through activation of the PERK-CHOP [41].
Mitochondrial dysfunction
Increased hepatic fat would increase hepatic fat oxidation with increased mitochondrial respiration [42,43]; however, decreased efficiency of respiratory chain complexes with greater mitochondrial uncoupling and leaking activity was found in patients with NAFLD [43,44]. Chronic mitochondrial dysfunction in the state of lipid overload led to excessive leakage of electrons from mitochondrial respiratory complexes, leading to oxidative stress [45]. Voltage-dependent anion channel acted as an early sensor of lipid toxicity, and its glycogen synthase kinase 3-mediated phosphorylation status controlled outer mitochondrial membrane permeabilization in hepatocytes with fat accumulation [46]. Exposure of hepatocytes to saturated FFAs caused mitochondrial depolarization, cytochrome c release, and increased ROS production [47]. Furthermore, intake of SFAs can affect the composition of mitochondrial membrane and decrease the efficiency of the respiratory transport chain, resulting in increased oxidative stress and chronic liver injury [48]. Peng et al. [49] found that hepatic cardiolipin and ubiquinone accumulated in NAFL and the levels of acylcarnitine increased with NASH, and proposed that increased levels of cardiolipin and ubiquinone may help to preserve mitochondrial function in early NAFLD; however, mitochondrial function eventually fails with the progression of NASH, leading to increased acylcarnitine. Moreover, SFAs increased ceramide synthesis in hepatocytes [50], which correlated with hepatocyte death via mitochondrial failure [51,52].
Lysosomal dysfunction
It has been shown that hepatic activity of lysosomal acid lipase and lysosomal acidification, which are markers of lysosomal dysfunction, are decreased in patients with NAFLD [53,54]. Both steatotic- and asparagine-treated hepatocytes showed reduced lysosomal acidity and retention of lysosomal calcium [55]. FFAs resulted in Bax translocation to lysosomes and lysosomal destabilization with the release of cathepsin B into the cytosol, leading to nuclear factor kappa B-dependent tumor necrosis factor alpha expression and apoptosis [56,57]. Lysosomal permeabilization and cathepsin B redistribution into the cytoplasm occurred several hours prior to mitochondrial dysfunction [47]. Furthermore, autophagy could sequester intracellular proteins and organelles in double-membrane vesicles (autophagosomes) to lysosomes for degradation. Autophagy in the regulation of intracellular lipid stores is called macrolipophagy [58]. Toxic fatty acids inhibited autophagic flux with reduction in lipophagy, which could lead to cell injury [59].
Oxidative stress and apoptosis
The main mechanisms of fatty acid-induced damage are oxidative stress and increased pro-inflammatory cytokines [2]. These insults from the ER stress, mitochondrial dysfunction, and oxidative stress in the hepatocytes after lipid accumulation could cause lipotoxicity, leading to apoptosis, necroptotis, or pyrotosis [60,61]. Saturated FFAs can also induce apoptosis through intrinsic and extrinsic pathways. The oxidative stress and ER stress induced by accumulated fatty acids can activate CHOP and JNK, and then upregulate Bim, Bax, and Bak, leading to the release of cytochrome C and caspase 9-associated apoptosis. In addition, death receptor pathways, including TRAIL/TRAIL receptor, tumor necrosis factor-α (TNFα)/TNF receptor 1 (TNFR1), and Fas ligand/Fas, were noted to be activated by FFAs on hepatocytes [62].
DEVELOPMENT OF NASH FIBROSIS
Liver fibrosis is the most important risk factor for liver cancer in patients with NAFLD and decompensated cirrhosis [63]. In patients with NAFLD, age and comorbidities, such as hypertension, overweighted, and diabetes mellitus, are risk factors for progression of fibrosis [64-66].
Lipotoxic damage in hepatocytes would release cytokines and chemokines, and then activate innate and adaptive immune cells, including macrophages, dendritic cells, lymphocytes, and neutrophils, leading to an inflammation cascade [67]. Damaged hepatocytes also release extracellular vesicles containing exosomes, microparticles, and apoptotic bodies. These vesicles, containing signaling proteins, sonic hedgehog (Hh), lipids, mRNAs, non-coding RNAs, and DNA, can induce inflammation, fibrosis by activating non-parenchymal cells, and recruitment of immune cells [68,69]. Meanwhile, apoptotic bodies can also be engulfed by stellate cells and subsequently induce HSC activation, which increases the expression of α–smooth muscle actin, transforming growth factor β (TGFβ), and collagen type I [70]. Moreover, the Hh pathway was not only activated in hepatocytes, leading to macrophage recruitment and progression of inflammation [71], but it also induced epithelial-to-mesenchymal transitions in ductular-type progenitors [72]. Cholangiocytes and natural killer T cells also activated Hh-osteopontin pathway and promoted fibrogenic responses of HSCs in NASH [73,74].
Toxic fatty acids were able to directly affect Kupffer cells (KCs) and HSCs, which may contribute to the activation of inflammation and fibrosis. Palmitic acids activated toll-like receptor (TLR) 2 and TLR4 in macrophages with the induction of inflammatory signaling [75]. KCs exhibited a pro-inflammatory response with elevated levels of TNFα, interleukin (IL)-6, and IL-1β after treatment by palmitic acids [75]. Palmitate induced ER stress and actin stress fiber formation in activated HSCs. Oleate induced the inflammatory signal and decreased cytoskeleton proteins in activated HSCs [76]. Free cholesterol was increased in patients with NAFLD, and the accumulation of free cholesterol in HSCs sensitized these cells to TGFβ-induced activation, leading to exaggerated liver fibrosis in NASH [10,77].
Insulin exerts profibrogenic activity. Insulin itself induces HSC mitogenesis and collagen synthesis [78,79]. However, insulin enhances the expression of smooth muscle actin-α in quiescent, but not in activated HSC through the PI3K/Akt-p70S6K pathway [80].
HSCs express PNPLA3 and membrane-bound O-acyltransferase domain-containing protein 7 (MBOAT7) [81,82]. Increased PNPLA3 expression reduces lipid droplet content in HSCs [81]. Autophagy promotes loss of lipids in HSCs to provide energy for HSC activation [83]. PNPLA3I148M can interfere with retinol production and release of HSCs by affecting the retinyl-palmitate lipase activity, which may promote fibrosis progression [81]. The MBOAT7 rs641738 T allele was associated with lower protein expression in the liver, and changes in plasma phosphatidylinositol species were consistent with decreased MBOAT7 function [82]. Hepatocyte-specific knockout of Mboat7 increased hepatic fibrosis with increased total lysophosphatidylinositol levels [84], which could promote the initiation of HSC activation by stimulating G-protein receptor 55 [85]. TM6SF2E167K was associated with higher risk of advanced fibrosis in NAFLD patients [86]. Furthermore, the gene encoding for the hepatic hydroxysteroid 17-beta dehydrogenase 13 (HSD17B13) regulated hepatic phospholipids and chronic liver injury in NAFLD patients [87-89]. The HSD17B13 rs72613567 variant led to the loss of enzyme function, contributing to reduced inflammation and fibrosis in the liver [88]. In addition, the HSD17B13 rs72613567 variant affected retinol metabolism by reducing the activity of retinyl-palmitate lipase, mediating antifibrotic and anti-inflammation effects [90].
Hypomethylation or hypermethylation of genes involved in the wound-healing process in NAFLD could be used to distinguish between patients with mild fibrosis from those with severe fibrosis in NAFLD. Hypermethylation at specific CpGs within TGFβ1 and PDGF, and hypomethylation at specific CpGs within peroxisome proliferator-activated receptor (PPAR) α and PPARδ in patients with mild fibrosis, were found [91].
ORGAN-ORGAN INTERACTION LEADING TO PROGRESSION OF NASH AND ITS FIBROSIS
Gut-liver axis
Compared with healthy adults, patients with NAFLD had a higher proportion of Firmicutes in the intestine, and the relative numbers of Bacteroidetes, Enterobacteriaceae, and Ruminococcaceae families were reduced [92,93]. Dysbiosis may disturb gut barriers, and bacteria and its products from the gut, such as endotoxin and cytokines, that promote inflammation, could enter the liver through blood, and activate the immune response in the liver and increase liver inflammation and fibrosis [94,95]. Compared with healthy people, patients with NAFLD had dysbiosis and increased intestinal permeability, and patients with steatohepatitis were observed to have endotoxemia [96,97]. Low-dose endotoxin stimulations were able to produce steatohepatitis in obese mice [98]. Conversely, blocking the signals caused by the immune system to recognize bacteria and its products effectively improved the severity of steatohepatitis [99,100]. Bacterial products and translocated lipopolysaccharide stimulated the hepatic innate immune system through TLR4 signaling, predominantly on HSCs and KCs [101]. TLR4-mediated stimulation of HSCs led to HSC activation and KC activation [102]. In turn, KCs produced TGFβ, stimulating fibrogenesis, and the proinflammatory cytokines, propagating hepatic inflammation. KCs also produced reactive oxygen species, leading to the generation of other reactive nitrogen species and local tissue damage [102,103]. Mice fed with high-fat diet for only 1 week underwent a diet-induced dysbiosis, driving the damage on gut vascular barrier and causing bacterial translocation into the liver [104]. However, only 42.1% of patients with steatohepatitis had elevated endotoxin levels [105] and 39.1% of fatty liver patients had increased intestinal permeability [106]. Therefore, bacterial translocation due to gut barrier impairment may play a partial role in the development and progression of NAFLD and its fibrosis.
Some metabolites in the blood and feces have been found to rely on bacterial synthesis, including choline and choline-related metabolites, bile acids, short-chain fatty acids (SCFAs), and ethanol, which may contribute to the pathogenesis of fatty liver. In animal experiments, the gut microbiota of mice fed with high-fat diet could convert choline into trimethylamine, reduce the bioavailability of choline, and produce a phenomenon similar to choline-deficient diet, leading to decreased excretion of VLDL from liver cells and increased liver fat accumulation [107]. Intestinal dysbiosis would increase the deoxycholic acid:chenodeoxycholic acid ratio, reduce the activation of farnesoid X receptor (FXR) signaling in the liver, reduce insulin sensitivity, increase glycogen and lipogenesis, and reduce fatty acid oxidation in the liver [108]. At the same time, gut dysbiosis also inhibited FXR, reduced the secretion of fibroblast growth factor (FGF) 15/19, leading to fatty liver [109]. SCFAs, such as acetate, propionate, and butyrate, are the products of bacterial fermentation of carbohydrate in the gut [110]. SCFAs in the intestine enter the liver through the portal vein, and acetate and propionate are precursors for fatty acid synthesis and gluconeogenesis, promoting liver fat accumulation [111]. Furthermore, SCFAs bind to G protein-coupled receptors of intestinal neuroendocrine L cells to secrete peptide YY and glucagon-like peptide-1 (GLP-1), which promote nutrient absorption and liver fat generation [112]. Butyrate may activate the AMP-activated protein kinase (AMPK) pathway in the liver, leading to the inhibition of oxidative stress and inflammation, upregulation of fatty acid oxidation, downregulation of fat synthesis genes, and reduced hepatosteatosis [113]. Interestingly, patients with NAFLD and severe hepatic fibrosis had more acetate in the stool, while those with milder severity of NAFLD had more SCFAs in butyrate and propionate [114]. Moreover, the concentration of ethanol in the blood of patients with NAFLD increased, and the bacteria Proteobacteria, which could produce ethanol, also tended to increase in patients with steatohepatitis. Ethanol destroys the tight binding protein of the intestinal wall, increases the intestinal permeability, and increases the endotoxin entering blood and the liver, leading to liver inflammation [115].
Adipose tissue-liver axis
Adipose tissues secrete adiponectin, leptin, and some proinflammatory cytokines, such as IL-6 and TNFα, which would influence the liver. Adiponectin binds to adiponectin receptors 1 and 2, respectively activates AMPK and PPAR-alpha pathways in the liver, and stimulate glucose use and fatty acid oxidation [116,117]. Adiponectin also increases carnitine palmitoyltransferase I activity, enhances hepatic fatty acid oxidation, and decreases the activities of acetyl-CoA carboxylase and fatty acid synthase [118]. However, adiponectin produced mainly from white adipose tissue is decreased in NASH patients [119]. When obesity develops, leptin secreted from white fatty tissue is increased to inhibit appetite and increase fatty acid oxidation [120]. However, in obese individuals, leptin resistance develops, and the increased leptin would exert proinflammatory activity. The serum leptin levels are positively associated with the severity of liver inflammation and fibrosis [121,122]. Leptin augments the endothelin-1-induced contraction of HSCs [123]. Adipocytes also secrete TNFα [124], which can increase insulin resistance and have pro-inflammatory effects [125]. TNFα increased the gene expression of Mcp1, Tgfb1, and Timp1 in hepatocytes, and the Tnf knockout improved glucose tolerance and significantly reduced the prevalence of hepatic steatosis and fibrosis in mice, indicating that TNFα plays a role in the development and progression of NASH [126]. IL-6 can be secreted from adipocytes, which can then increase the macrophage infiltration of adipose tissue [127]. IL-6 infusion induces hepatic insulin resistance through increased adipose tissue lipolysis [128]. These data suggest that IL-6 is involved in the pathogenesis of hepatic insulin resistance.
Renin-angiotensin system (RAS)
Hypertensive patients with biopsy-proven NAFLD on baseline RAS blockers had less advanced hepatic fibrosis [129]. Recently, a large retrospective study showed that angiotensin-converting enzyme inhibitors/angiotensin receptor blockers were associated with lower risk of hepatocellular carcinoma and cirrhotic complications in patients with NAFLD [130]. These data suggested a beneficial effect of RAS blockers in NAFLD. Transgenic hypertensive rats overexpressing the mouse renin gene with elevated levels of tissue angiotensin II developed hepatic steatosis, inflammation, and fibrosis [131]. The mice lacking the renin gene fed with high-fat diet had decreased liver fat [132]. Aliskiren, a direct renin inhibitor, reduced hepatic steatosis in high-fat diet-fed mice and fibrosis in mice fed with methionine-choline-deficient diet [133,134]. When renin or prorenin binds to the (pro)renin receptor (PRR), in addition to increasing the production and role of angiotensin (ANG II dependent pathway), it activates TGFβ, plasminogen activator inhibitor-1 (PAI-1), fibronectin, and collagen I independently from Ang II (ANG II independent pathway) [135-137]. Our group found that PRR contributed to liver fibrosis and HSC activation, and its down-regulation attenuated liver fibrosis through inactivation of the ERK/TGFβ1/Smad3 pathway [138]. These results indicate that renin and prorenin can directly activate renin (pro) receptor-related intracellular signaling pathways, including ERK, TGFβ, cyclooxygenease2, fibronectin, collagen I, and PAI-1 independently of angiotensin II to induce fibrosis. Moreover, Ren et al. [139] used N-acetylgalactosamine modified antisense oligonucleotides to suppress PRR expression in hepatocytes of high-fat diet-fed C57BL/6 mice, and found that PRR inhibition reduced acetyl-CoA carboxylase and pyruvate desorption hydrogenase protein expression. This change reprogrammed liver lipid metabolism, resulting in reduced lipid synthesis and increased fatty acid oxidation. As a result, liver PRR suppression attenuated dietinduced obesity and fatty liver [139]. The proposed pathogenesis that is involved from steatosis to fibrosis in patients with NAFLD is shown in Figure 1.

Progression of hepatic steatosis to inflammation and fibrosis in liver. Both metabolic and genetic factors contribute to the formation of hepatic steatosis. Fat accumulation in hepatocytes leads to organelles dysfunction and lipotoxicity. Then, oxidative stress species or signaling molecules are transmitted through extracellular vesicles or diffusion, activating other parenchymal and non-parenchymal cells, which subsequently causes inflammatory cascades, steatohepatitis, and liver fibrosis. On the other hand, gut-derived bacterial end-products, metabolites, gut hormones, adipose tissue-derived cytokines or adipokines, and renin-angiotensin-system all contribute to the progression from steatosis to inflammation and fibrosis. SFA, saturated fatty acid; TG, triglyceride; ER, endoplastic reticulum; HH-OPN, Hedgehog-osteopontin; KC, Kupffer cell; HSC, hepatic stellate cell.
PROGRESSION OF NASH TO HCC
NASH is now the most common risk factor for HCC in the United States [140]. The potential pathways linking NASH to HCC include chronic inflammation of the liver [141], alternations in immune response, lipid metabolism and gut microbiome [142], and genetic factors. Enhanced IL-6 and TNF production during NAFLD cause hepatic inflammation and activation of the oncogenic transcription factor STAT3 [143]. ER stress contributes to NASH-driven hepatic tumorigenesis via TNFR1 [144]. The hepatic oxidative DNA damage was increased in patients with NASH who developed HCC [145]. The unconventional prefoldin RPB5 interactor-induced DNA damage in hepatocytes triggered inflammation via T helper 17 lymphocytes and interleukin 17A, contributing to NASH and HCC development [146]. Furthermore, NAFLD caused a selective loss of intrahepatic CD4(+) but not CD8(+) T lymphocytes, which led to accelerated hepatocarcinogenesis [147]. Neutrophil infiltration was characterized in NASH-HCC and can exist in both tumor promoting and suppressing states [148]. Fatty acid accumulation increased junctional protein associated with coronary artery disease, leading to the activation of Yes-associated protein 1 and tumor growth [149]. Dysregulated mammalian target of rapamycin stimulated sphingolipid and glycerophospholipid synthesis, leading to steatosis and HCC [150]. In NASH-driven HCC, metabolic reprogramming mediated by the downregulation of carnitine palmitoyltransferase 2 enables HCC cells to escape lipotoxicity and promotes hepatocarcinogenesis [151]. MicroRNA-21 can promote hepatic lipid accumulation and cancer progression by interacting with the sHbp1-p53-Srebp1c pathway [152]. The intestinal dysbiosis, gut permeability changes, and lipopolysaccharides translocation to the liver in NASH may increase secretion of the epiregulin growth factor, which triggers tumor hepatocyte proliferation [153]. Moreover, carriage of the PNPLA3 rs738409 C>G polymorphism is associated with a greater risk of NASH-HCC [154].
TREATMENT FOR NASH
Non-pharmacological therapy
Lifestyle modification
Lifestyle changes by eating less and exercising more to achieve weight loss remain the cornerstone of clinical care. Hypocaloric diet with a reduction of body weight decreased total body fat, visceral fat, and intrahepatic lipid content [155]. Some existing guidelines suggest restriction of energy by 1,200–1,500 kcal/day or a reduction of 500–1,000 kcal/day to achieve weight loss [1,156-158]. Weight reduction is beneficial for both non-obese (3–10%) [159] and obese patients (≥0%) [160,161]. Other dietary compositions that may be beneficial for NAFLD includes omega-3 PUFA and coffee. Omega-3 PUFA has been shown to increase insulin sensitivity [162] and ameliorate steatohepatitis in experimental studies [163,164]. One meta-analysis involving nine studies with 355 patients showed decreased liver fat in patients with PUFA treatment [165]. Coffee is not only associated with a reduced risk of NAFLD but also decreased risk of liver fibrosis among patients with NAFLD [166,167]. Regular exercise helps to enhance the effects of diet modifications. Physical activity with a target at least 150 min/week of moderate-intensity or 75–150 min/week of vigorous-intensity aerobic exercise is suggested [1,156-158]. Both aerobic and resistance exercises reduce the hepatic fat content [168,169]. In addition, the intensity of exercise may be more important than the duration or total volume [170]. In conclusion, lifestyle interventions to promote weight loss, which include both diet and exercise, are proven therapeutic strategies to improve fatty liver disease.
Surgery
Bariatric surgery provides sustained and durable weight loss and improving obesity-related diseases [171,172]. Currently, the most commonly performed bariatric procedures are laparoscopic sleeve gastrectomy (LSG) and Roux-en-Y gastric bypass. Two meta-analyses showed that bariatric surgery resulted in a biopsy-confirmed resolution of steatosis (56–66%), inflammation (45–50%), ballooning degeneration (49–76%), and fibrosis (25–40%), as well as reduction of NAFLD activity score (NAS) [173,174]. A higher rate of improvement in steatosis and hepatic fibrosis was observed in Asian countries compared to non-Asian countries [174]. In addition, bariatric surgery was associated with decreased progression of NAFLD to cirrhosis [175] and reduced risks of any cancer and obesity-related cancer in NAFLD patients with severe obesity, particularly in cirrhotic patients [176]. However, new or worsening cases of NAFLD were found in 12% of patients after bariatric surgery [173]. Bariatric surgery was associated with a significantly lower risk of incident major adverse liver outcomes (2.3% vs. 9.6% at 10 years) and major adverse cardiovascular events (8.5% vs. 15.7% at 10 years), as compared with non-surgical management [177].
Endoscopic therapy
Endoscopic bariatric therapies, including intragastric balloons (IGB), endoscopic sleeve gastroplasty (ESG), duodenal mucosal resurfacing (DMR), and duodenal-Jejunal bypass liner (DJBL), were recently introduced as less invasive modalities to treat obesity and metabolic comorbidities. In a meta-analysis, improvement in steatosis and NAS were seen in 79.2% and 83.5% of patients receiving IGB, respectively [178]. Improvement of fibrosis for 1.5 stage by MR elastography was seen in 50% of patients with NAFLD after IGB placement [179]. ESG reduced the body weight by up to 15% and improved hepatic steatosis and fibrosis at 2 years of follow-up in obese patients with NAFLD [180]. Studies for efficacy and safety of ESG (NCT03426111; NCT04653311) and the comparison of ESG vs. LSG (NCT04060368) in patients with NASH are ongoing. DMR has been shown to reduce alanine aminotransferase, aspartate aminotransferase, and fibrosis-4 scores in patients with diabetes mellitus [181]. Recently, an observational study of 32 obese patients with diabetes mellitus who underwent DJBL showed improved non-invasive markers of steatosis and NASH, but not fibrosis. The role of DJBL on NAFLD needs to be further evaluated [182].
Fecal microbiota transplantation
Some studies have suggested that fecal transplantation helps ameliorate steatohepatitis [183,184]. A randomized controlled trial (RCT) using allogenic fecal microbiota transplantation (FMT) from lean vegan donors for patients with NAFLD through duodenal infusion found that there was no significant improvement in NAS, steatosis, and fibrosis scores. However, they observed a trend of improving necro-inflammatory scores and beneficial changes in hepatic gene expression and plasma metabolites involved in inflammation and lipid metabolism following allogenic FMT [185]. Another RCT using allogenic FMT via endoscopic duodenal infusion in patients with NAFLD found that FMT did not improve insulin resistance and hepatic steatosis but reduced small intestinal permeability at 6 months of follow-up [186].
Pharmacological therapy
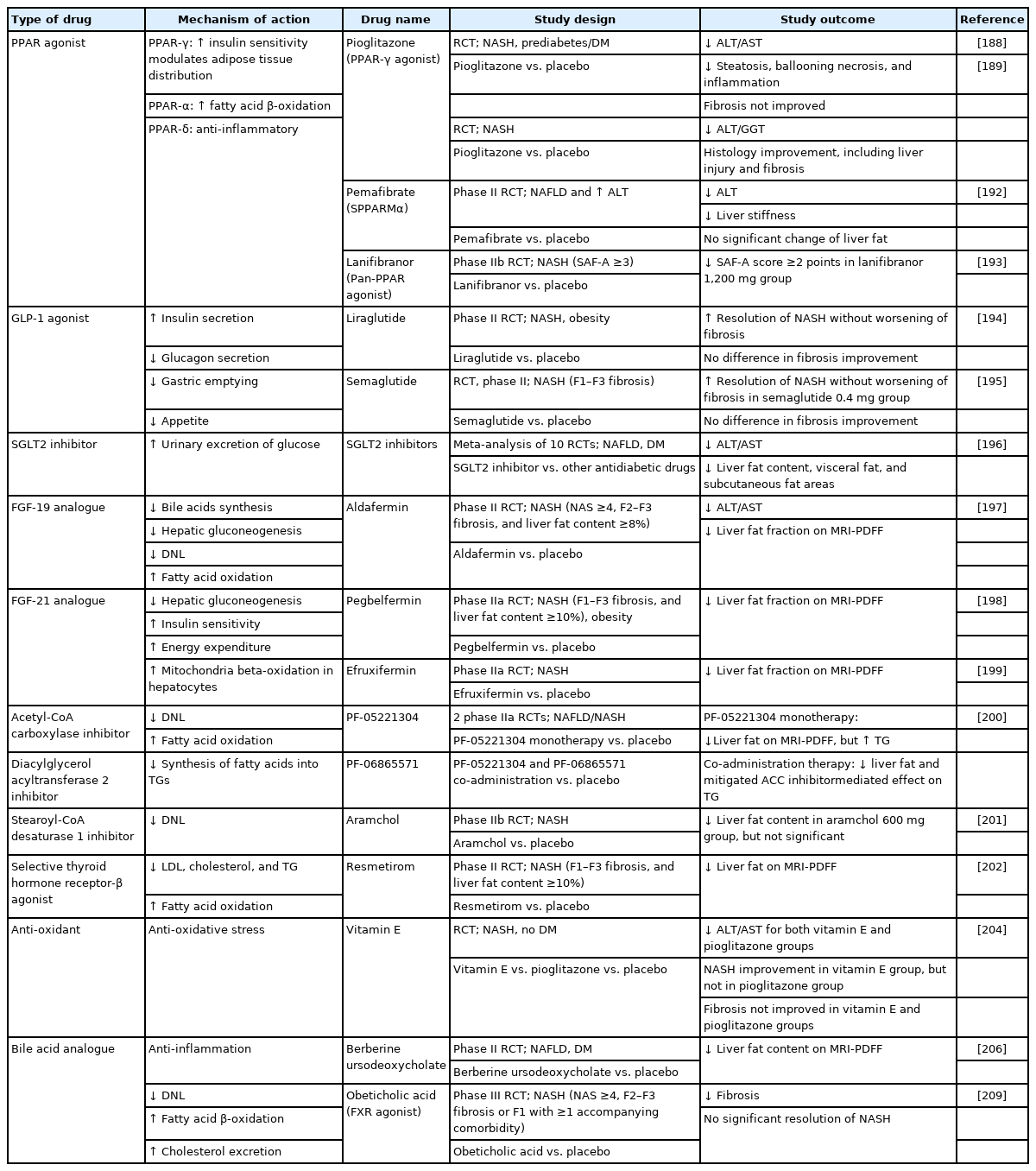
The pharmacological agents predominantly target the following four mechanisms: 1) hepatic fat accumulation; 2) oxidative stress, inflammation, and apoptosis; 3) gut-liver axis, including bile acids, gut microbiomes, and metabolic endotoxemia; and 4) hepatic fibrosis [187]. The agents targeting different pathways are described below, and those with promising results are summarized in Table 1.
Agents targeting hepatic fat accumulation
Pioglitazone, a PPAR γ agonist, improved hepatic steatosis, inflammation, and hepatocellular ballooning [188,189]. Similar effects were found in Asian NASH patients [190]. In the phase 3 RESOLVE-IT trial, Elafibranor, a dual PPAR α/δ agonist, failed to achieve NASH resolution [191]. Pemafibrate, a selective PPAR α modulator, did not decrease liver fat but caused a significant reduction in fibrosis for 6.2% of magnetic resonance elastography-based liver stiffness [192]. Lanifibranor, a pan-PPAR agonist, significantly decreased the steatosis-activity-fibrosis activity score for at least 2 points in 55% of the patients at 24 weeks [193].
GLP-1 agonists increase insulin secretion, inhibit glucagon secretion, delay gastric emptying, and decrease appetite. NASH resolution was observed in 39% of patients who received liraglutide for 48 weeks and in 59% of patients who received semaglutide for 72 weeks [194,195]. However, fibrosis improvement was insignificant in both studies.
Sodium-glucose cotransporter 2 (SGLT2) inhibitors increase the urinary excretion of glucose. A meta-analysis of 10 RCTs showed that SGLT2 inhibitors can reduce aminotransferases and hepatic fat [196].
FGF19 and FGF21 are endocrines that regulate energy homeostasis. Aldafermin, a FGF19 analogue, led to reductions of liver fat content and a trend toward fibrosis improvement [197]. Pegbelfermin and efruxifermin are long-acting, recombinant analogues of human FGF21, and both have shown effects of reducing liver fat [198,199].
Two phase IIa trials investigated the effects of acetyl-coenzyme A carboxylase (ACC) inhibitor monotherapy (PF-05221304) and combination with a diacylglycerol O-acyltransferase 2 (DGAT2) inhibitor (PF-06865571). Both PF-05221304 monotherapy and co-administration with PF-06865571 reduced liver fat content [200].
Stearoyl-coenzyme A desaturase 1 (SCD-1) is a key enzyme that catalyzes the biosynthesis of monounsaturated fatty acids. A phase IIb trial (ARREST trial) showed that aramchol (a liver-targeted SCD-1 inhibitor) 600 mg did not cause a significant reduction in liver fat content. Nevertheless, the observed change in liver histology and biochemical improvement suggests a potential role of aramchol in treating NASH and fibrosis [201].
Thyroid hormone receptor-β (THR-β) is predominantly expressed in the hepatocytes. Resmetirom, a selective THR-β agonist, significantly reduced more than 30% of hepatic fat after 12 and 36 weeks of treatment in patients with NASH in phase II trial [202].
Agents targeting oxidative stress, inflammation, and apoptosis
Vitamin E, an antioxidative agent, demonstrated benefits on hepatic decompensation and transplant-free survival in patient with NASH [203]. The PIVENS study, which compared the effects of vitamin E, pioglitazone, and placebo in NASH patients without diabetes, showed that vitamin E (800 international units/day), but not pioglitazone, significantly improved NASH [204].
Apoptosis signaling kinase 1 (ASK1) promotes apoptosis, inflammation, and fibrosis in the liver. However, selonsertib, an ASK1 inhibitor, failed to improve fibrosis in NASH patients with bridging fibrosis or compensated cirrhosis [205].
Berberine ursodeoxycholate is an ionic salt of berberine and ursodeoxycholic acid. It reduced 4.8% of liver fat and improved glycemic control as well as liver enzymes in patients with NASH and diabetes [206].
Agents targeting gut-liver axis
In a phase IIb study, obeticholic acid (OCA), a FXR agonist, improved liver histology in 21% of NASH patients [207]. In patients with NASH and diabetes, OCA demonstrated the effects of increasing insulin sensitivity and reducing markers of liver inflammation as well as fibrosis [208]. In the interim analysis of a phase III trial, both 10-mg and 25-mg doses of OCA improved fibrosis (18% and 23%, respectively), but the NASH resolution endpoint was not met [209]. This study is ongoing to assess the clinical outcomes.
Agents targeting liver fibrosis
Caspase is a protease that is associated with apoptosis and inflammation in the liver. However, emricasan, a pan-caspase inhibitor, did not improve fibrosis or resolution of NASH [210]. Besides, for patients with NASH-related cirrhosis and severe portal hypertension, emricasan did not improve hepatic venous pressure gradient (HVPG) or liver-related outcomes [211].
In a phase IIb CENTAUR trial, a 2-year study, cenicriviroc, a dual C-C chemokine receptor types 2 and 5 antagonist, achieved ≥1-stage of fibrosis improvement without worsening of NASH after 1 year of treatment compared to placebo (20% vs. 10%) [212]. And a great proportion (60%) of the patients who achieved fibrosis response at the first year maintained fibrosis reduction at the second year [213]. The long-term impact of cenicriviroc on fibrosis needs to be further investigated.
Belapectin, a galectin-3 inhibitor, did not significantly reduce HVPG or fibrosis in patients with NASH, cirrhosis, and portal hypertension; however, in a subgroup of patients without esophageal varices, belapectin reduced HVPG as well as the development of esophageal varices [214].
Information about the ongoing phase III clinical trials of promising drugs on phase II studies are listed in Table 2.
Combination therapy
NAFLD is a multifactorial disease, and combining therapies with different targets may have synergistic effects [215]. Cilofexor (FXR agonist) plus firsocostat (ACC inhibitor) led to improvements in NASH activity compared to placebo, or single agent in patients with bridging fibrosis and cirrhosis [216]. Semaglutide with firsocostat and/or cilofexor showed greater improvements in liver steatosis and liver biochemistry compared to semaglutide alone [217]. Combining ACC inhibitors and DGAT2 inhibitors reduced liver fat content and mitigated the side effect of elevated serum TGs [200].
PERSPECTIVES
As understanding of mechanisms of NASH and its fibrosis increases, more therapies will be introduced and tested in clinical trials. The pathogenesis of NASH and fibrosis is complex; therefore, it would be difficult to treat the disease using just one therapy. Combination therapy is the focus in the future development of treatment. Furthermore, better care of extra-hepatic complications of NASH, novel biomarkers for diagnosis, risk stratification and treatment responses, and more clinical trials in Asian groups should also be well researched and developed.
Notes
Authors’ contribution
KC Lee and PS Wu drafted the manuscript and HC Lin revised the manuscript. All the authors read and approved the final version.
Conflicts of Interest
The authors have no conflicts to disclose.
Acknowledgements
This study was supported in part by the grants from the Ministry of Science and Technology (110-2628-B-075-016; 111-2314-B-075-051-MY3) Taiwan.
Abbreviations
ACC
acetyl-coenzyme A carboxylase
AMPK
AMP-activated protein kinase
ANG
angiotensin
ASK1
apoptosis signaling kinase 1
CHOP
C/EBP homologous protein
DGAT2
diacylglycerol O-acyltransferase 2
DJBL
duodenal-Jejunal bypass liner
DMR
duodenal mucosal resurfacing
DNL
de novo lipogenesis
ER
endoplasmic reticulum
ESG
endoscopic sleeve gastroplasty
FFA
free fatty acid
FGF
fibroblast growth factor
FMT
fecal microbiota transplantation
FXR
farnesoid X receptor
GCKR
glucokinase regulatory protein
GLP-1
glucagon-like peptide-1
HCC
hepatocellular carcinoma
Hh
hedgehog
HSC
hepatic stellate cell
HSD17B13
hydroxysteroid 17- beta dehydrogenase 13
HVPG
hepatic venous pressure gradient
IGB
intragastric balloons
IL
interleukin
IRE1
inositol-requiring enzyme 1
IRGM
immunity-related GTPase M
KC
Kupffer cell
LSG
laparoscopic sleeve gastrectomy
MBOAT7
membrane-bound O-acyltransferase domain-containing protein 7
miRNA
microRNA
NAFLD
non-alcoholic fatty liver disease
NAS
NAFLD activity score
NASH
non-alcoholic steatohepatitis
NEFA
non-esterified fatty acid
OCA
obeticholic acid
PAI-1
plasminogen activator inhibitor-1
PERK
protein kinase R (double-stranded RNA-activated protein kinase)-like ER kinases
PNPLA3
patatin-like phospholipase domaincontaining 3
PPAR
proliferator-activated receptor
PRR
(pro)renin receptor
PUFA
polyunsaturated fatty acid
RAS
renin-angiotensin system
RCT
randomized controlled trial
SCD-1
stearoyl-coenzyme A desaturase 1
SCFA
short-chain fatty acid
SFA
saturated fatty acid
SGLT2
sodium-glucose cotransporter 2
SNP
single nucleotide polymorphism
SREBP1c
sterol receptor binding protein 1-c
TG
triglyceride
TGF
transforming growth factor
THR-β
thyroid hormone receptor-β
TLR
toll-like receptor
TM6SF2
transmembrane 6 superfamily 2
TNF
tumor necrosis factor
TNFR1
TNF receptor 1
UPR
unfolded protein response
VLDL
very-low-density lipoprotein
XBP-1
X-box-binding protein 1
ω6
omega-6