Nonalcoholic fatty liver disease versus metabolic-associated fatty liver disease: Prevalence, outcomes and implications of a change in name
Article information
Abstract
Nonalcoholic fatty liver disease (NAFLD) affects about a third of the world’s adult population and is a major public health concern. NAFLD is defined by the presence of hepatic steatosis and the absence of other causes of liver disease. As NAFLD is closely associated with the presence of the metabolic syndrome, several experts have called for a change in nomenclature from NAFLD to metabolic-associated fatty liver disease (MAFLD) to better reflect the underlying pathophysiology of NAFLD as a metabolically driven disease and shift to a “positive” diagnostic criteria rather than one of exclusion. Recent studies have suggested that the global prevalence of MAFLD is higher than that of NAFLD, and patients with MAFLD have more metabolic comorbidities compared to those with NAFLD. Emerging data also suggest that all-cause and cardiovascular mortality may be higher in MAFLD compared with NAFLD. In this synopsis, we discuss differences in clinical features, prevalence and clinical outcomes between NAFLD and MAFLD. In addition, we highlight the advantages and disadvantages of a name change from NAFLD to MAFLD from the perspective of the scientific community, care providers and patients.
INTRODUCTION
The concept of nonalcoholic fatty liver disease (NAFLD) was first introduced in the early 1980s when histopathological features similar to alcohol-associated liver disease were observed in the absence of alcohol consumption [1,2], Currently, NAFLD affects 29–33% of the global population [3-5] and has emerged as a major concern for public health. The American Association for the Study of Liver Diseases (AASLD) defines NAFLD based on the presence of hepatic steatosis on imaging or biopsy and requires the exclusion of other causes of chronic liver disease [6]. NAFLD is classified into two categories: nonalcoholic fatty liver (NAFL), the benign, non-progressive form of NAFLD, and nonalcoholic steatohepatitis (NASH), the inflammatory form which may progress to cirrhosis and hepatocellular carcinoma (HCC) [6-9].
NAFLD is strongly associated with the presence of the metabolic syndrome and the rise in NAFLD closely mirrors the obesity epidemic [10,11]. Modelling studies have estimated that by 2030, 10% of the global population is projected to have diabetes [12], and nearly 50% of the USA population is projected to be obese [13]. While NAFLD affects a substantial proportion of the population, there is currently no Food and Drug Administration (FDA)-approved treatment for NAFLD, and liver transplantation is often the only treatment for patients with decompensated NASH cirrhosis [14,15]. Additionally, the presence of NAFLD results in a significantly higher risk of extrahepatic end organ damage, along with associated psychological stress [16]. Unfortunately, despite the severe morbidity and mortality burden from NAFLD, the global awareness of NAFLD remains low, particularly amongst non-hepatologists and primary care physicians who are at the frontlines managing metabolic diseases [17]. A recent international survey reported that there is a lack of emphasis on NAFLD in national health agendas in the majority of countries [18], and one third of countries scored zero on the preparedness index [19].
Meanwhile, substantial progress has been made in understanding the underlying disease mechanism of NAFLD [20]. In turn, this has lead to a call from several experts for a change in nomenclature from NAFLD to metabolic associated fatty liver disease (MAFLD), to better reflect the underlying pathophysiology of NAFLD as a metabolically driven disease [21,22]. In addition, these experts cited the absence of defined clinical criteria for a “positive” diagnosis of this disease in the traditional definition of fatty liver. Two position papers sought to integrate current understanding of patient heterogeneity captured under the acronym NAFLD and provide suggestions on terminology that can better reflect the pathogenesis of the disease [21,22]. The experts in these two papers believe that the name MAFLD more accurately describes the disease as a metabolic disorder and shifts it from a disease of exclusion to one of inclusion [21,22]. In this review, we highlight the differences in clinical features, prevalence and outcomes between NAFLD and MAFLD including the prevalence and impact of heaptic steatosis on the natural history of patients with other concurrent liver disease such as hepatitis B. In addition, we discuss the benefits, disadvantages and implications of a change in name from NAFLD to MAFLD.
DEFINITION OF NAFLD VERSUS MAFLD
An expert panel defined MAFLD as evidence of hepatic steatosis with obesity, type 2 diabetes, or ≥2 factors associated with evidence of metabolic dysfunction (Table 1) [21-23]. Importantly, the exclusion of alternative causes of chronic liver disease, such as alcohol or viral hepatitis, are no longer required to diagnose MAFLD.
DIFFERENCES IN CLINICAL FEATURES BETWEEN NAFLD AND MAFLD
Risk factors
Recent data demonstrate significant differences in clinical characteristics between NAFLD and MAFLD [4]. In a recent systematic review and meta-analysis, NAFLD-MAFLD patients were more metabolically unhealthy compared to NAFLD individuals [4]. MAFLD patients had significantly higher odds of having diabetes, chronic kidney disease (CKD) and/or hypertension as compared to NAFLD patients [4]. A recent USA study used data from the Third National Health and Nutrition Examination Survey and categorized patients into three groups: non-MAFLD NAFLD, NAFLD-MAFLD, and non-NAFLD MAFLD [24]. Patients in the NAFLD-MAFLD and non-NAFLD MAFLD groups were older and had more metabolic risk factors than the non-MAFLD NAFLD group. In addition, the non-NAFLD MAFLD group were more likely to have hypertension, elevated aminotransaminases and advanced fibrosis when compared with the NAFLD-MAFLD and non-MAFLD NAFLD groups.
Prevalence
A recent meta-analysis estimated that the global prevalence of MAFLD and NAFLD to be 39% and 33%, respectively [4]. Nearly 30% to 40% of patients with hepatitis B have concurrent hepatic steatosis [25,26], and these patients were previously excluded from the definition of NAFLD. Under the new diagnostic criteria for MAFLD (Table 1), those with hepatic steatosis, concomitant metabolic dysfunction, and additional cause(s) of chronic liver disease may be included in the new definition. Only 81% of patients met criteria for both MAFLD and NAFLD, with the difference contributed by the exclusion of a substantial proportion of lean and non-obese NAFLD from MAFLD and exclusion of patients with other concomitant liver disease from NAFLD. Lean NAFLD presents a distinct subtype that accounts for 5.1% of the global population and are known to have significantly less metabolic comorbidities [27].
Outcomes
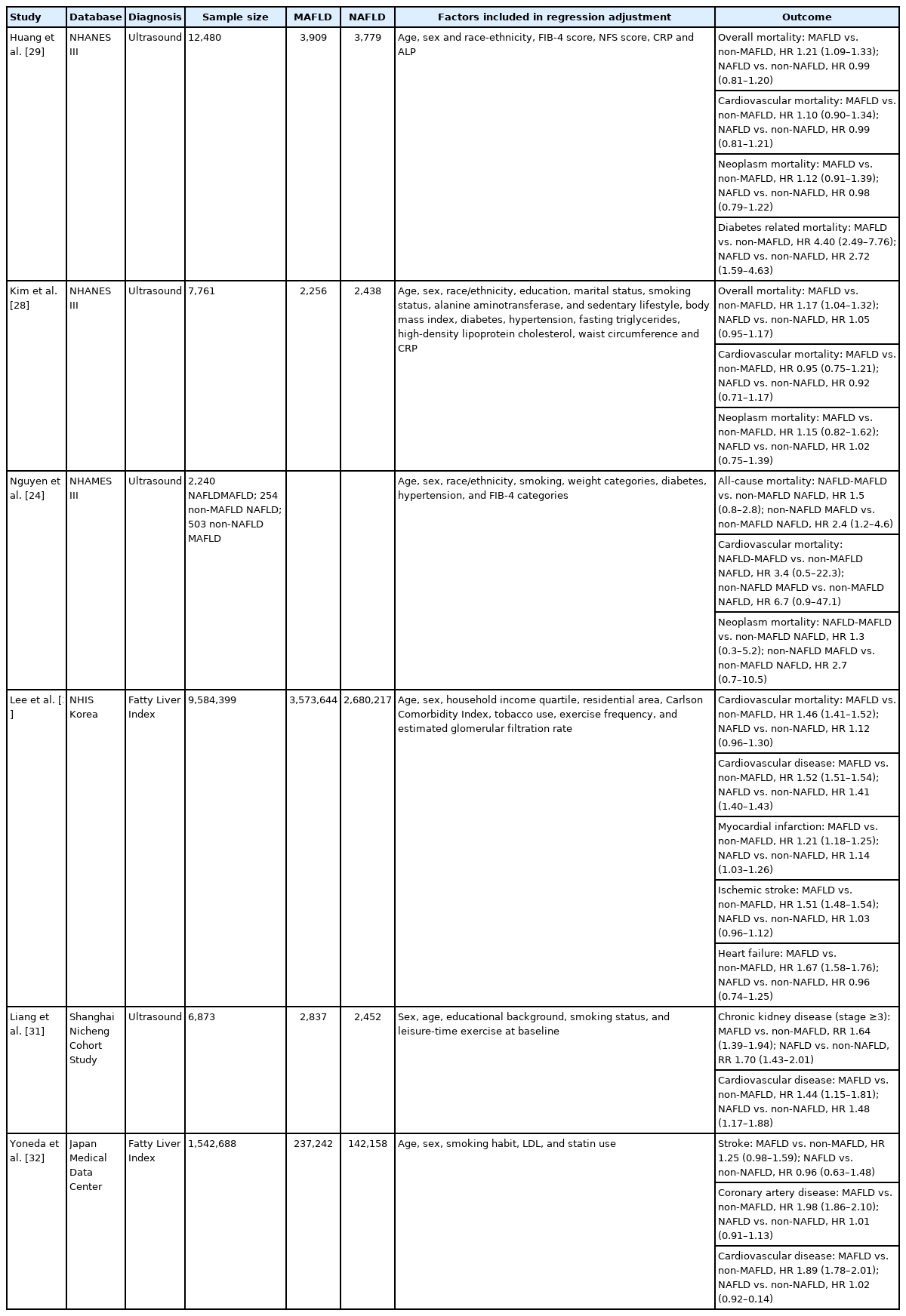
Non-NAFLD MAFLD was found to have a higher risk of all-cause mortality (hazard ratio [HR], 2.40; 95% confidence interval [CI], 1.2–4.6; P=0.01) after adjusting for age, sex, race/ethnicity, smoking, viral hepatitis, FIB-4 and weight compared to non-MAFLD NAFLD (Figs. 1, 2). Cardiovascular disease-related mortality (HR, 6.70; 95% CI, 0.9–47.1; P=0.06) was also larger in non-NAFLD MAFLD compared to non-MAFLD NAFLD after adjusting for demographic factors and provides evidence that the presence of MAFLD may be a better predictor of adverse events in the presence of hepatic steatosis [24]. The increased risk of overall mortality in the presence of MAFLD as compared to non-MAFLD was confirmed by a latter study also using the same database [28]. Interestingly, cardiovascular and cancer-related mortality were not significantly different between MAFLD and NAFLD, suggesting that cause(s) other than cardiovascular or cancer such as liver related cause may be responsible to the higher overall mortality with MAFLD [28,29]. Using data from the National Health Insurance Service in South Korea, the presence of MAFLD (vs. non-MAFLD) was associated with a significant increase in cardiovascular mortality (HR, 1.46; 95% CI, 1.41–1.52) but not the presence of NAFLD compared to non-NAFLD (HR, 1.12; 95% CI, 0.96–1.30) [30]. The risk of developing systemic end organ damage including cardiovascular disease, stroke and CKD was marginally higher in MAFLD compared to NAFLD (Table 2) after adjusting for confounders [31,32].
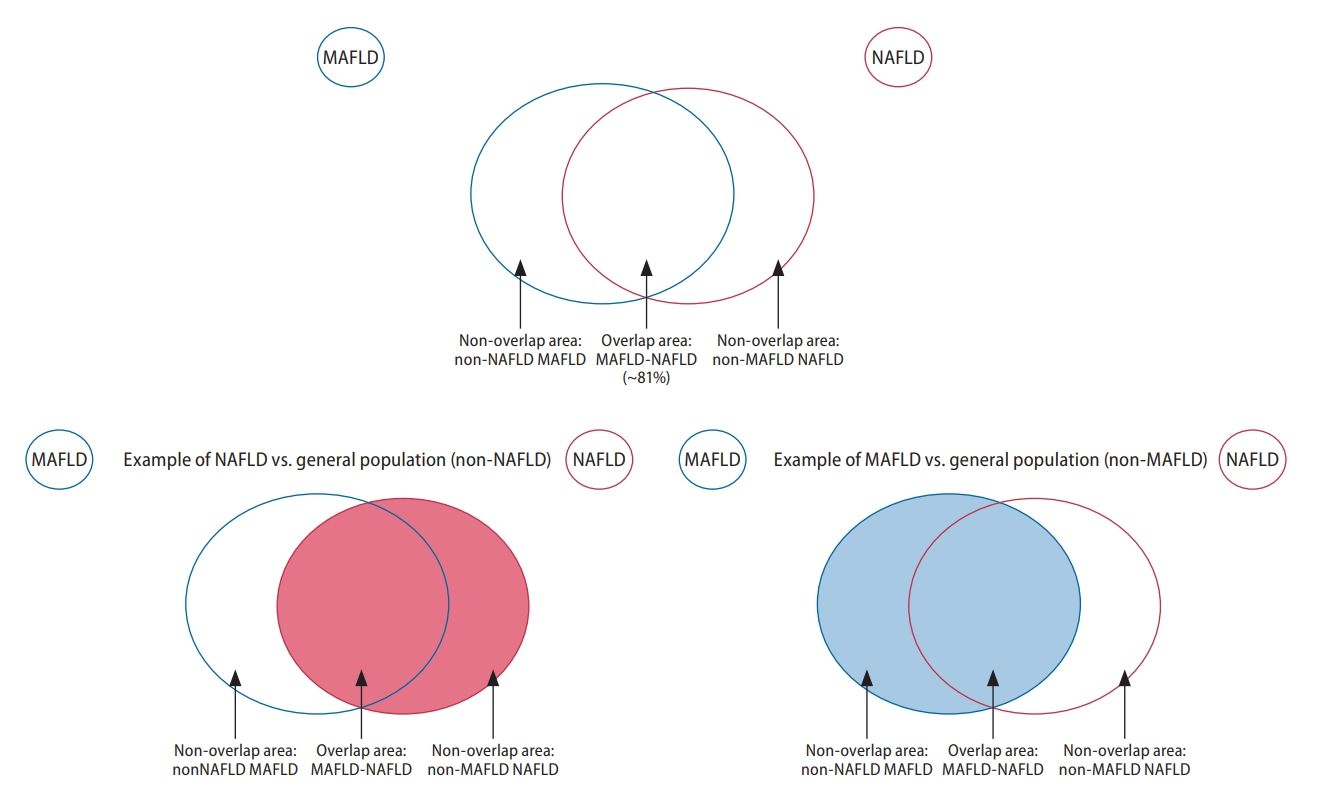
Graphical illustration of groups included in the analysis of overlapping conditions. MAFLD, metabolic associated fatty liver disease; NAFLD, nonalcoholic fatty liver disease.
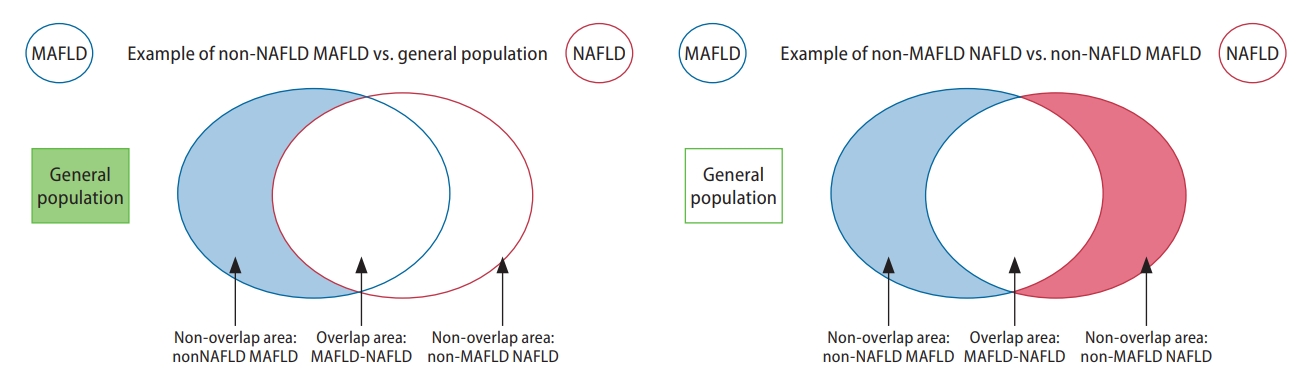
Graphical illustration of groups included in the analysis of non-overlapping conditions. MAFLD, metabolic associated fatty liver disease; NAFLD, nonalcoholic fatty liver disease.
As the diagnosis of MAFLD does not require the exclusion of another concurrent liver disease such as chronic hepatitis B, we noted here the prevalence, characteristics, and outcome of patients with fatty liver and chronic hepatitis B, a disease that affects about 290 million people worldwide [25,33,34]. A recent meta-analysis inclusive of 54 studies (28,648 patients) estimated that about one-third of patients with chronic hepatitis B (CHB) have concurrent hepatic steatosis and that the presence of hepatic steatosis was not significantly associated with significant fibrosis (odds ratio [OR], 0.87; 95% CI, 0.54–1.30; 20 studies; 6,232 patients). More recent studies have specifically examined the association between MAFLD and liver histopathology in patients with concomitant viral hepatitis. One retrospective analysis of 773 patients with biopsy-confirmed MAFLD found a higher proportion of patients with fibrosis stage 2–4 among MAFLD patients with concurrent viral hepatitis compared with patients with only MAFLD, but this study only included 40 patients with viral hepatitis which was also mixture of patients with hepatitis B virus (HBV) and patients with hepatitis C virus (HCV) infection [35]. Another recent biopsy study included a much larger cohort of patients with concomitant HBV infection (359 of the total cohort of 417; 86%) found that HBV infection was independently associated with higher grades of inflammation and fibrosis despite having an inverse association with the severity of hepatic steatosis [36]. However, the results of these two biopsy studies should be interpreted with caution as patients undergoing liver biopsy as part of their routine care are often highly selected, so the results may not be generalizable to the general population of patients with MAFLD and HBV infection. Additionally, the sample size of patients in one comparative group for both of these studies was very small (only 40 patients with MAFLD and viral hepatitis in one study and only 58 patients with MAFLD alone in the other). Regarding concurrent HCV infection and MAFLD, one study found that 43% (n=321) of 744 patients with chronic HCV infection had hepatitc steatosis on liver biopsy [37]. The study also found that concurrent MAFLD (vs. HCV infection without MAFLD) was independently associated with fibrosis stage 2–4.
In regards to long-term clinical outcomes, the presence of MAFLD-HBV was found to be associated with a higher risk of overall mortality, HCC and decompensation compared to non-MAFLD HBV [38]. HCC patients affected by both HBV and MAFLD undergoing curative resection were more likely to have recurrence compared to HCC patients with HBV infection without MAFLD [39]. However, while some studies found that patients with HBV and hepatic steatosis have worse outcomes [40,41], others have found that the presence of steatosis in HBV was asscociated with lower risk of cirrhosis, hepatocellular carcinoma, and higher chance of hepatitis B surface antigen seroclearance [42-47]. Thus, further studies are needed to elucidate the relationship between hepatic steatosis, metabolic derangement, and long-term clinical complications [42,48].
ADVANTAGES OF A CHANGE IN NAME FROM NAFLD TO MAFLD
From the perspective of the scientific community
The advantages and disadvantages of a change in nomenclature are summarized in Figure 3. Significant advancements of clinical science have offered insights into the mechanisms between NAFLD, diabetes and obesity [49]. Though metabolic dysfunction is the key driver of hepatic steatosis, the current definition of NAFLD does not account for factors that are major predictors of metabolic dysfunction, such as obesity and diabetes [50]. The heterogeneity within NAFLD with respect to its primary metabolic drivers represent an important impediment to the discovery of efficacious therapies. MAFLD is a better reflection that metabolic dysregulation is the mechanism underlying the disease [51].
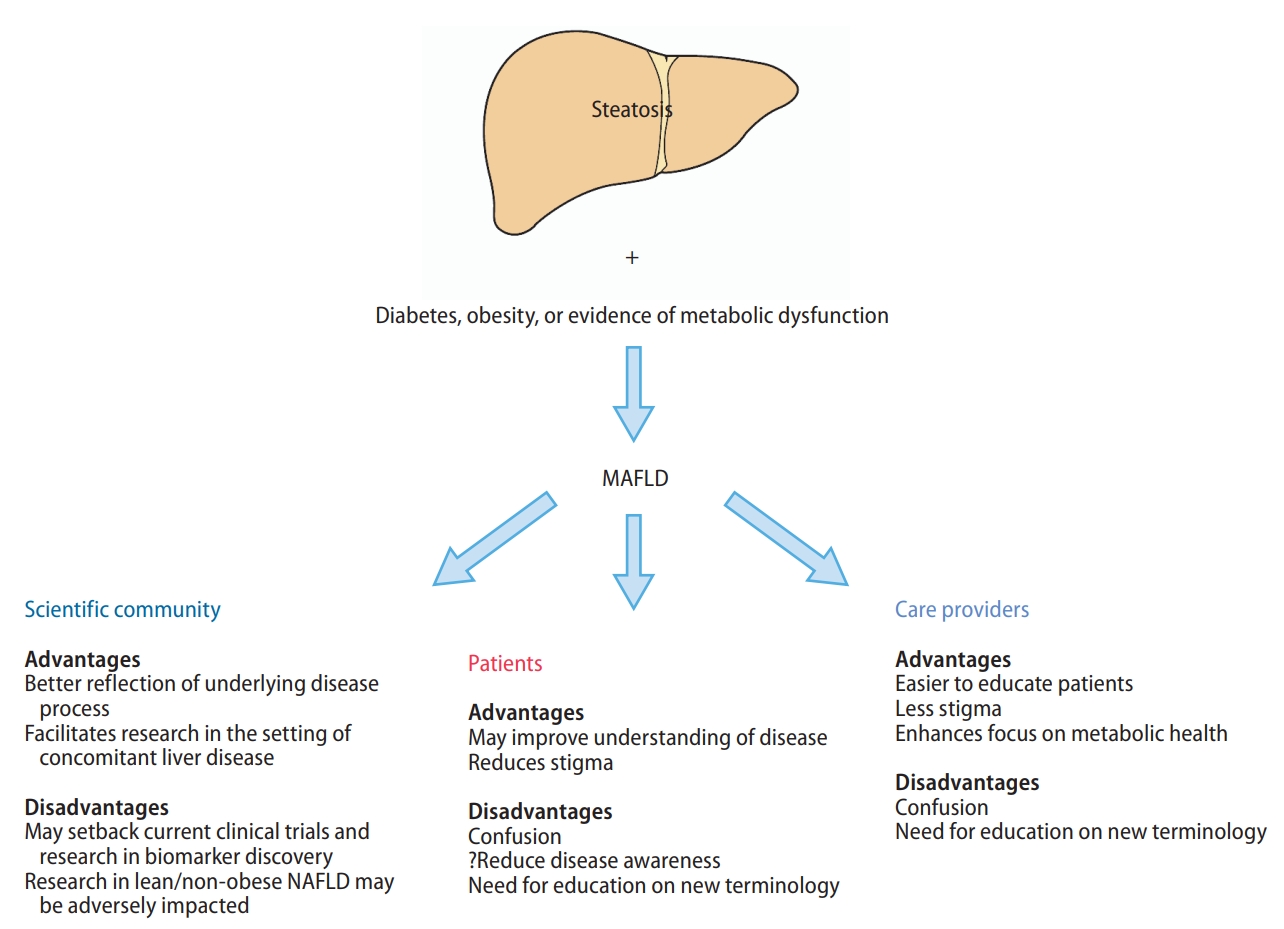
Advantages and disadvantages of a change in name from NAFLD to MAFLD. MAFLD, metabolic associated fatty liver disease; NAFLD, nonalcoholic fatty liver disease.
Another major advantage of the change in nomenclature to MAFLD is the removal of the need to exclude other chronic liver diseases when studying patients with hepatic steatosis, such as alcohol and viral hepatitis, which would need to be excluded before a diagnosis of NAFLD can be made. For example, as one-third of the 290 million world HBV population may have hepatic steatosis [25], almost 100 million patients with concomitant CHB and hepatic steatosis would not be included under the definition of NAFLD but would be included under the umbrella term of MAFLD. Therefore, switching of nomenclature to MAFLD may help promote research and collaboration in conditions with concomitant hepatic steatosis and other causes of liver disease, such as viral hepatitis or alcohol-associated liver disease [52]. Additionally, the threshold for exclusion of significant alcohol use as required in the definition of NAFLD is often not clear. The current definition of “safe” alcohol consumption is generally defined as an intake not amounting to 30 g/day in men or 20 g/day in women, but this threshold has been hotly contested, especially in the setting of NAFLD [24,53]. A systematic review by the Global Burden of Disease confirmed that there was a lack of consensus as to what constitutes a “safe” limit in alcohol consumption [54]. Additionally, the measure of alcohol use at both patient- and population-level is often compromised by substantial recall bias despite formal quantitative alcohol consumption questionnaires as developed by the National Institute on Alcohol Abuse and Alcoholism [55,56]. As a result, a shift to use the MAFLD nomenclature may help reduce the heterogeneity and confusion for the research communities as well as clinicians caring for patients with hepatic steatosis associated with both metabolic disease and significant alcohol consumption.
Finally, since the disease burden of fatty liver disease is vast, increased engagement among the care provider community beyond liver specialists is needed, and the nomenclature that directly indicates “metabolic” derangement can help engage care providers in other disciplines such as cardiology and endocrinology in the screening, diagnosis, and management of patients with fatty liver and metabolic disease, namely MAFLD. A recent meta-analysis of over 12 million people found a significantly higher prevalence of a wide range of systemic complications in individuals with MAFLD compared to those without MAFLD, including cardiovascular disease, extrahepatic maglinancy and CKD [57]. This emphasizes the importance of multidisciplinary engagement in the management of patients with MAFLD.
From the perspective of patients and providers
NAFLD is often underdiagnosed and consequently is associated with presentation with more advanced disease and increased mortality [58]. In fact, a USA population-based study found that only 5% of people with NAFLD are aware of having a liver disease [59]. The use of the name NAFLD may contribute to poor disease awareness and understanding among patients with the disease, as patients often want to know what their disease is, not what it is not [60]. A change of nomenclature to MAFLD can remove the burden of an exhaustive work up to rule out all other causes of liver disease and having the word “metabolic” in the new nomenclature may make the disease mechanism more intuitive to both patients and care providers alike, and thus facilitating better disease understanding and management. In certain regions such as the Middle East and North Africa, the association of the name alcohol with NAFLD may also result in stigma and confusion [60], which may lead to delayed diagnosis and a higher proportion of patients being diagnosed with advanced liver disease and decompensation.
DISADVANTAGES OF A CHANGE IN NAME FROM NAFLD TO MAFLD
From the perspective of the scientific community
The change in name from NAFLD to MAFLD has been endorsed by the Chinese Society of Hepatology [61], Arabic Association for the Study of Diabetes and Metabolism [62], the Latin American Association for the Study of the Liver [63] and the Asia Pacific Association for the Study of the Liver [23]. A recent letter comprising over 1,000 signatories representing various professional bodies and physicians including hepatologists, endocrinologists, primary care physicians, nephrologists and cardiologists endorsed the change of definition to MAFLD [64]. However, to date, neither AASLD nor the European Association for the Study of the Liver have endorsed the change in nomenclature [65].
Several experts in the field have expressed concerns that a premature change in definition from one suboptimal name to another without a comprehensive assessment and consensus from all stakeholders may result in greater challenges to biomarker discovery and drug development [66]. While there are currently no FDA approved treatments for NASH, several therapies such as lanifibranor [67], semaglutide [67], and obeticholic acid [68] have demonstrated encouraging results. A sudden change in nomenclature may have a major impact the inclusion criteria for many ongoing clinical trials and may inadvertently result in delay to the approval of efficacious therapies for NAFLD. A change in name to MAFLD may also set back current progress in biomarker development, where studies have been mostly performed among patients with hepatic steatosis, without significant alcohol consumption, viral hepatitis, autoimmune liver disease, etc [69]. An example would be the discovery and development of the NIS4 which was created specifically for NASH [70]. There is an increasing recognition in the field that non-invasive tests may be appropriate endpoints for clinical trials in NAFLD; therefore, a change in disease definition may substantially set back the progress made in recent years [66],
Another implication of the change of name to MAFLD may be a reduced focus on lean or non-obese NAFLD. A recent meta-analysis estimated that the non-obese NAFLD affects 12.1% of the global population and that 40% of the global NAFLD population are non-obese [27]. In addition, among people with non-obese NAFLD, 27% have diabetes compared to only 2.4% among the general population [27]. Lean people with NAFLD may also be at higher risk of disease progression [71,72], and 6% of non-obese persons with NAFLD had metabolic disease, a proportion that is similar to that of obese people with NAFLD (61%) [73]. Further studies characterizing non-obese MAFLD are required as prior studies have suggested that non-obese NAFLD people may have higher mortality than obese people with NAFLD [71-75]. While MAFLD provides a greater focus on extrahepatic comorbidities associated with fatty liver, it potentially might result in an overemphasis on the systemic comorbidities that may result in a lack of focus on the liver itself, even though the higher risk of complications and disease could well be attributed to the presence of systemic dysregulation, rather than from the liver [57].
From the perspective of patients and care providers
In recent years, the term NAFLD has started to gain awareness among patients, primary care physicians and non-hepatology specialists [66]. Many patients with NAFLD are diagnosed by primary care physicians and non-hepatology specialists such as endocrinologists and cardiologists. A change in nomenclature from NAFLD to MAFLD may result in confusion and potentially result in even poorer disease awareness, potentially setting back the progress made in recent years.
CONCLUSION
Prior to any change in the nomenclature of the disease, it is vital that all stakeholders, including international liver societies, hepatologists, scientists, patient advocacy organizations, the bio-pharmaceutical industry, regulatory agencies, and policy makers meet and come to a consensus. Ultimately, any change in name needs to have clear advantages to patients that outweigh the potential disadvantages and consensus discussion needs to include mechanisms to address such disadvantages, such as setting back current progress in biomarker discovery and drug development for NAFLD. Any new definition would require intensive re-education of patients and care providers, particularly primary care physicians and non-hepatologist specialists. Finally, a greater emphasis must be placed on multidisciplinary and preventive care in light of the high prevalence of systemic metabolic diseases among people with metabolic-associated fatty liver disease.
Notes
Authors’ contributions
Study design: DQY, JKH, MHN; Data interpretation and review/revision of the manuscript: All authors; Study concept and study supervision: MHN; All authors approved the final draft of the manuscript as well as the authorship list.
Conflicts of Interest
Mindie H. Nguyen: Research support: Pfizer, Enanta, Gilead, Glycotest, Vir, B.K. Kee Foundation, National Cancer Institute. Advisory board/consulting: Janssen, Spring Bank, Gilead, Novartis, Bayer, Eisai, Eli Lilly, Exact Sciences, Laboratory of Advanced Medicine, Helio Health, Intercept.
Daniel Q. Huang: Research support: Exxon Mobil-NUS Research Fellowship for Clinicians, NMRC Research Training Fellowship. Advisory board/consulting: Eisai.
Other authors have no disclosures.
Abbreviations
AASLD
American Association for the Study of Liver Diseases
CHB
chronic hepatitis B
CI
confidence interval
CKD
chronic kidney disease
FDA
Food and Drug Administration
HBV
hepatitis B virus
HCC
hepatocellular carcinoma
HCV
hepatitis C virus
HR
hazard ratio
MAFLD
metabolic associated fatty liver disease
NAFL
nonalcoholic fatty liver
NAFLD
nonalcoholic fatty liver disease
NASH
nonalcoholic steatohepatitis
NHIS
National Health Insurance Service
OR
odds ratio