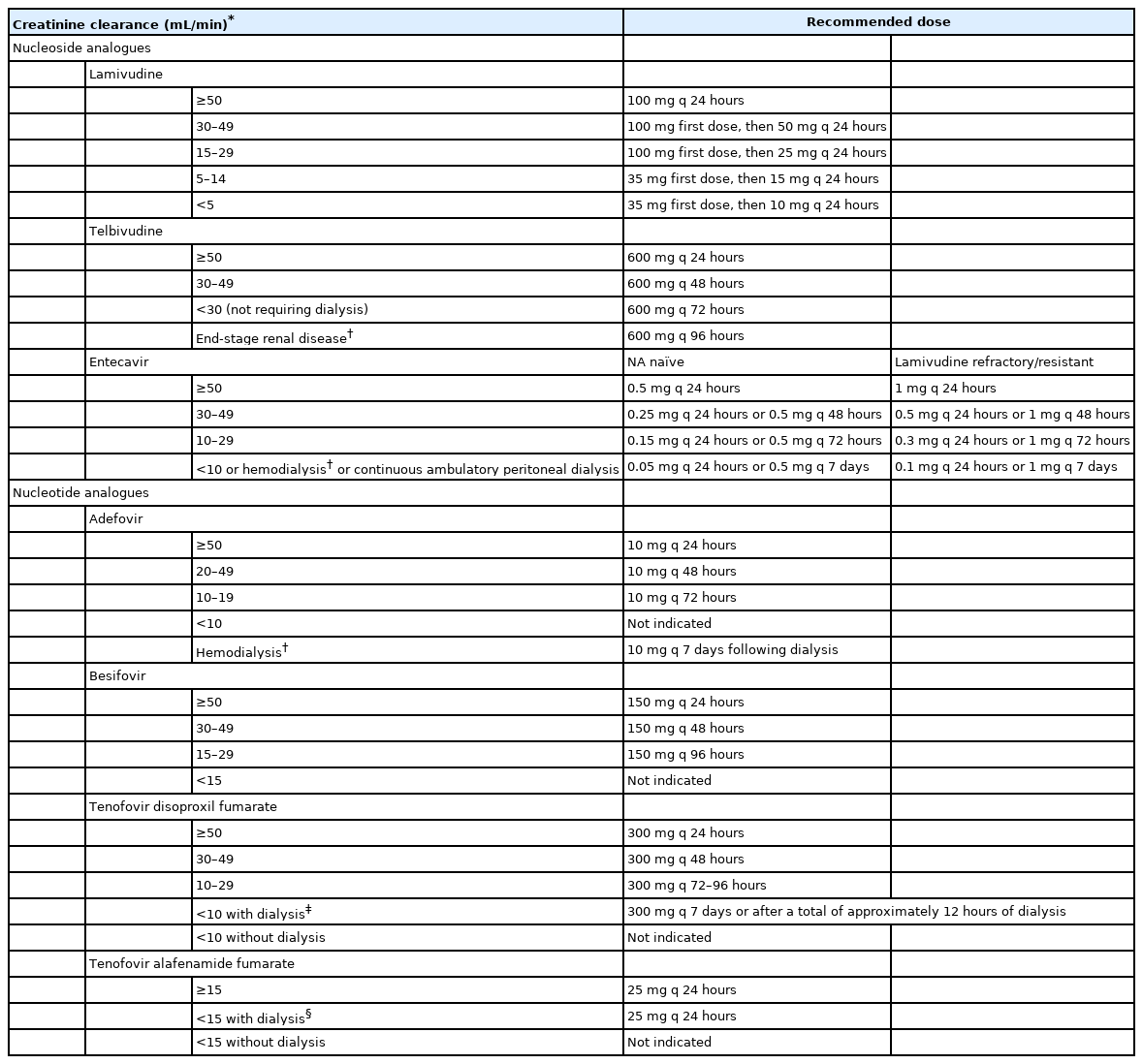
KASL clinical practice guidelines for management of chronic hepatitis B
Article information
INTRODUCTION
Chronic hepatitis B (CHB) is a major cause of chronic liver diseases in Korea and remains a threat to public health due to its high morbidity and disease-related mortality. Since the latest version of clinical practice guidelines of CHB in 2018 by the Korean Association for the Study of the Liver (KASL), many studies related to hepatitis B virus (HBV) have been published, raising the need to revise the recommendations reflecting the most up-to-date information on management of CHB. The guidelines are intended to provide useful information and medical guidance for clinicians responsible for diagnosing and treating Korean patients with CHB. The current version differs from the published 2018 KASL guidelines, which revised the entire part about CHB, in that it primarily focuses on the 12 major clinical topics that require updated medical information and the latest knowledge. In addition to the natural course of CHB, treatment indication, cessation of antiviral therapy, and management for special populations covered in the previous version of the guidelines, this version will establish new topics on ‘Emerging Markers of HBV Infection’ and ‘New Drugs for Functional Cure,’ which have recently showed advancements for diagnosis and treatment of CHB. Recommendations on parts of CHB not included in this guideline are noted in the 2018 guidelines.
The current practice of managing hepatitis B has become more complex as many targeted and immune therapies are being widely used for systemic treatments of cancer patients with chronic HBV infection. In the updated guidelines, the reported rates of hepatitis B reactivation associated with these new drugs were added, and prophylactic antiviral treatment was recommended accordingly. In transplant settings of the liver and solid organs, the risk of and management against post-transplant hepatitis B recurrence were subclassified according to the status of HBV markers of donors and recipients. In particular, prophylactic management of patients undergoing hematopoietic stem cell transplantation who are at a very high rate of HBV reactivation was separately described. The 2022 guidelines summarized the emerging therapies for a cure of HBV and newly provided hepatocellular carcinoma (HCC) risk models and emerging markers of HBV for clinical utility. In addition, the guidelines updated the feasibility of cessation of antiviral drugs using new HBV markers and management of patients with bone and renal disease, co-infections with other hepatitis virus, and those at the so-called ‘grey zone.’
The Committee for 2022 KASL clinical practice guidelines for CHB, launched in accordance with the initiative of the Board of Directors of the KASL and approved by the council, was composed of nine hepatologists. The Committee searched newly published articles related to hepatitis B from PubMed, MEDLINE (up to 2021), KoreaMed, as well as abstracts and proceedings of international academic conferences and collected necessary data since publication of the 2018 guidelines in order to provide updated recommendations based on the latest data. The levels of evidence in the guidelines were classified by the revised Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system. The levels of evidence were based on the possibility of change in the estimate of clinical effect by further research and were described as high (A), moderate (B), or low (C). Classification of grades of recommendation were either strong (1) or weak (2), by the GRADE system, according to the level of evidence, generalizability, clinical effect of the research result, and socioeconomic aspects. Each recommendation is combined with the level of relevant evidence (A–C) and corresponding recommendation grade (1, 2) as follows: A1, A2, B1, B2, C1, and C2 (Table 1).
Expert opinions were solicited in cases of insufficient data to make definitive conclusions. In addition, specialists representing the Korean Society for Transplantation were invited to participate as external consultants, regarding the recommendations on hepatitis B in transplant settings. Each member of the committee was responsible for collecting, analyzing, and preparing the manuscript in their respective fields.
Health care utilization varies depending on race, region, institution, and economic conditions. The presented guidelines can differ from other regional guidelines as it reflected our unique medical conditions and research results. It is intended to provide practical and updated information for management of CHB patients. However, as the guidelines do not represent a standard treatment protocol, clinicians should keep in mind that the best management might vary by individual patient setting. The KASL will continue to update part or all of these guidelines based on publication of new study results. Thus, revision of the guidelines is deemed necessary for promoting the health of Korean CHB patients.
NATURAL HISTORY
CHB is defined as persistence of serum hepatitis B surface antigen (HBsAg) for more than six months. The natural course is divided into five clinical phases: immune-tolerant, hepatitis B e antigen (HBeAg)-positive immune-active, immune-inactive, HBeAg-negative immune-active, and HBsAg loss or resolved HBV infection (Table 2). The duration of these clinical phases can vary, and the sequences of phases might not be continuous in the patient. In addition, there can be grey zone in which the features do not correspond to any specific phase. Therefore, it is often insufficient to determine the clinical stage of infection or to decide antiviral treatment based on a single alanine aminotransferase (ALT) or HBV DNA test (Fig. 1) [1].
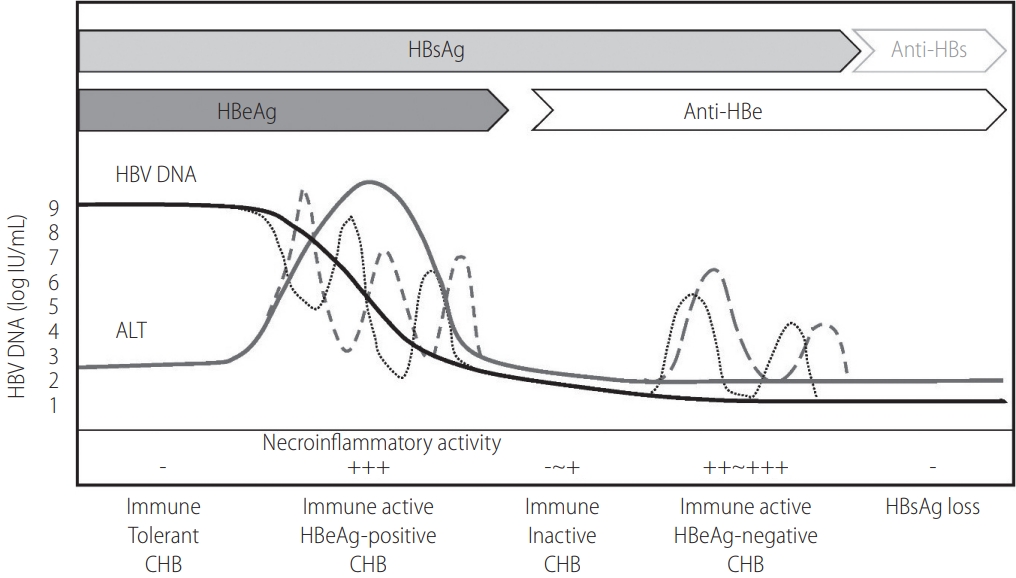
Natural course of chronic hepatitis B (CHB). HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen; anti-HBs, antibody to HBsAg; HBeAg, hepatitis B e antigen; anti-HBe, antibody to HBeAg; ALT, alanine aminotransferase.
Immunological features of CHB during the natural course
CHB, immune-tolerant phase
The immune-tolerant phase is generally related to vertical transmission and is characterized by HBeAg positivity, very high level of serum HBV DNA (generally ≥107 IU/mL), persistently normal level of ALT, and minimal or absence of hepatic necroinflammation [2,3]. In a follow-up of immune-tolerant CHB patients, serum ALT was elevated in 16% of cases, and the follow-up fibrosis stage was not different from the initial stage in those who remained immune-tolerant for 5 years [2]. In another study from Taiwan, 5% of 240 immune-tolerant CHB patients progressed to cirrhosis and did not develop HCC in 10 years of follow-up [4]. In a Korean multi-center study, 1% of 946 immune-tolerant CHB patients progressed to cirrhosis, and 1.7% developed HCC during 10 years of follow-up [5].
However, a small-scale in vitro study suggested that early hepatocarcinogenesis could be progressing even during the immune-tolerant phase, as was evident by a high level of HBV DNA integration and clonal hepatocyte expansion in liver tissues from immune-tolerant CHB patients as with immune-active CHB patients [6]. Recently, several studies reported that development of HCC was not uncommon in the immune-tolerant phase, although the studies included patients with serum HBV DNA <107 IU/mL, serum ALT >upper limit of normal (ULN), or high fibrosis index [7], necessitating further studies to clarify the criteria for discriminating patients in “true” immune-tolerant phase.
The immune-tolerant phase can last for more than three decades in patients infected with HBV genotype C due to late HBeAg seroconversion [8]. Therefore, many female patients infected with this genotype are in the immune-tolerant phase when they are of childbearing age, which can lead to vertical transmission of HBV to a child [9].
HBeAg-positive CHB, immune-active phase
With increasing age, most patients in the immune-tolerant phase experience immune responses to HBV. Such changes are due to increased response of cytotoxic T lymphocytes to hepatitis B core antigen (HBcAg) and HBeAg [10], resulting in destruction of infected hepatocytes. This phase is characterized by HBeAg positivity and fluctuating courses of serum ALT and HBV DNA levels [11,12]. Histological findings reveal moderate-to-severe necroinflammation [13]. There can be various stages of liver fibrosis according to severity of liver injury.
Once HBeAg seroconversion occurs, the natural course of the disease can have one of three clinical features: (1) repeated HBeAg reversion and seroconversion, (2) immune-inactive phase, or (3) HBeAg-negative, immune-active phase of CHB [14,15]. Typically, 10–40% of patients who experience seroconversion revert to an HBeAg-positive state and then experience recurrence of seroconversion at least once with progression of hepatitis activity [16,17]. In particular, reversion frequently occurs in patients with HBV genotype C, and the rate decreases with age [9]. Hepatic decompensation, which occurs in 5% of patients with acute exacerbation, can be fatal [18].
CHB, immune-inactive phase
Most patients who seroconvert during the immune-active phase progress to the immune-inactive phase, which is characterized by HBeAg negativity, antibody to HBeAg (anti-HBe) positivity, persistent normal ALT level, and HBV DNA level below 2,000 IU/mL [19-21]. Typical histological findings in the third phase are mild liver inflammation [19], and various stages of liver fibrosis can reflect previous liver injury [22].
This phase persists for an extended period in most patients but has a relatively good prognosis. However, an estimated 20% of such patients will revert to the HBeAg-negative or HBeAg-positive immune-active phase and can experience recurring periods of reactivation and inactivation throughout their lives, which can lead to cirrhosis or HCC [23,24].
HBeAg-negative CHB, immune-active phase
Approximately 20% of patients who experience HBeAg seroconversion during their immune-active HBeAg-positive phase progress to the immune-active HBeAg-negative phase, with HBV DNA level ≥2,000 IU/mL, increased ALT level, and active necroinflammation of the liver [14]. These patients show HBeAg-negativity because they harbor HBV variants in the precore (PC) or basal core promoter (BCP) regions of HBV DNA, resulting in failure or reduction of HBeAg production [25-28]. The immune-active HBeAg-negative phase is associated with older age and lower rates of prolonged spontaneous disease remission, and most patients in this phase will experience persistent hepatocellular inflammation and progress to hepatic fibrosis and cirrhosis [27,29,30]. Severe fluctuations of HBV DNA and ALT levels can make it difficult to differentiate these patients from those in the immune-inactive phase [31]. Therefore, HBV DNA and ALT levels should be monitored in every 3 months for at least 1 year in case of immune-inactive phase to find out the HBeAg-negative immune-active phase requiring antiviral treatment [32,33].
HBsAg loss phase
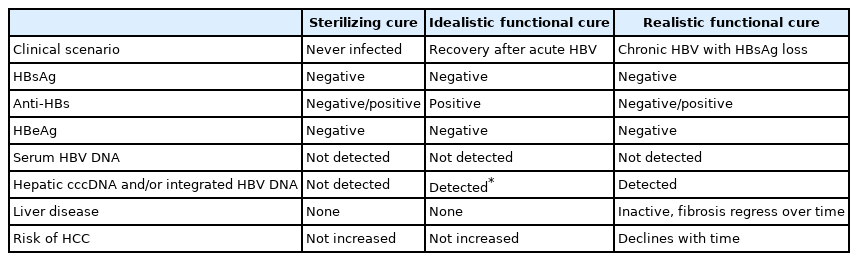
During the natural course of CHB, HBsAg loss is a very rare transition that indicates potential cure of HBV infection (Table 3) [34-37]. Complete cure, or “sterilizing cure,” of HBV infection implies seroclearance of HBsAg and HBV DNA as well as complete clearance of intrahepatic covalently closed circular DNA (cccDNA) and/or integrated HBV DNA. However, it is difficult to achieve these goals at present [37]. Accordingly, the realistic goal suggested is a “functional cure,” which refers to seroclearance of HBsAg and HBV DNA regardless of antibody to HBsAg (anti-HBs) [37]. Despite the presence of intrahepatic cccDNA and/or integrated HBV DNA, it is a successful immunological control state of CHB and therefore can be viewed as a concept similar to idealistic functional cure [36]. In certain circumstances, such as immunosuppression, the risk of HBV reactivation persists [38].
Patients in the immune-inactive phase subsequently progress to HBsAg loss or clearance phase at a rate of 1–2% annually [31,39,40]. According to Liaw’s prospective data, HBsAg loss occurs in 0.5% of CHB patients per year and 0.8% of asymptomatic chronic HBV carriers per year [35]. Korean patients reportedly experience a relatively low rate of HBsAg loss (0.4% annually) [41]. In a few patients, serum HBV DNA can be detected at a very low titer during this phase [42,43]. HBsAg loss is the state of functional cure and is associated with a reduced risk of cirrhosis. However, significant risk of HCC development remains even after HBsAg loss in male patients and in settings where HBsAg loss has been achieved late (presence of cirrhosis or age ≥50 years) [42,44].
HBsAg seroclearance is also achieved in a few patients on antiviral treatment, and the long-term clinical outcome compared with that of spontaneous HBsAg seroclearance is unclear [45,46]. Two studies showed no difference in HCC incidence between patients who achieved treatment-induced and spontaneous HBsAg seroclearance [47,48], yet another study reported a 5-year cumulative incidence of HCC of 0.9% and 3.9% in spontaneous and treatment-induced HBsAg seroclearance patients, respectively [49].
Grey zone
The natural course of CHB is divided into five phases with regard to clinical indicators such as ALT and HBV DNA – immune-tolerant, HBeAg-positive immune-active, immune-inactive, HBeAg-negative immune-active, and HBsAg loss. However, patients who do not fit into any of the usual clinical phases are considered to be in the “grey zone.” Approximately 30% of CHB patients are in the grey zone [50-52], and the prognosis and treatment of CHB patients in the grey zone are under study [52-57].
The decision whether to initiate antiviral therapy for patients in the grey zone is a clinically challenging question, and it is often difficult to make an accurate decision without the results of a liver biopsy. Treatment of patients in the grey zone is summarized in the ‘Treatment Indication’ session.
Risk factors that influence the natural history and progression of CHB
In the natural course of CHB, the cumulative incidences of cirrhosis and HCC were approximately 8–20%/year and 2–5%/year [58,59]. In Korea, the reported annual and 5-year accumulated incidences of cirrhosis are 5.1% and 23%, respectively, while those for HCC are 0.8% and 3% [60]. The risk factors for CHB progressing to cirrhosis or HCC can be divided into host, viral, and social-environmental factors (Table 4). For host factors, cirrhosis, persistent necroinflammation, old age, male gender, family history of HCC, and co-infection of other hepatitis virus or human immunodeficiency virus (HIV) affect the risk. High level of serum HBV DNA and/or serum HBsAg, HBV genotype C, and specific genotypic mutations are included as viral factors [61-67]. Social-environmental factors for progression to cirrhosis or HCC include alcohol consumption, metabolic syndrome, diabetes, obesity, and smoking [68-70]. In contrast, coffee [71-73], metformin [74], aspirin [75,76], and statins [77-84] exert protective effects against development of HCC.
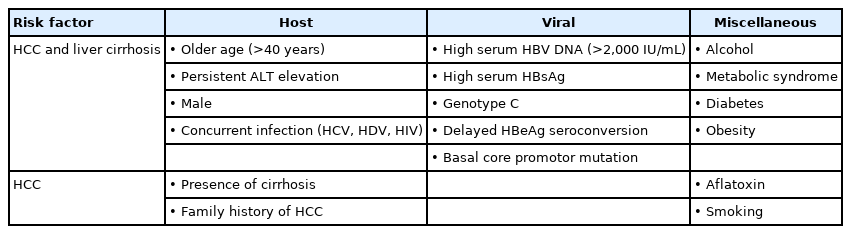
Factors associated with development of liver cirrhosis and hepatocellular carcinoma in persons with chronic hepatitis B
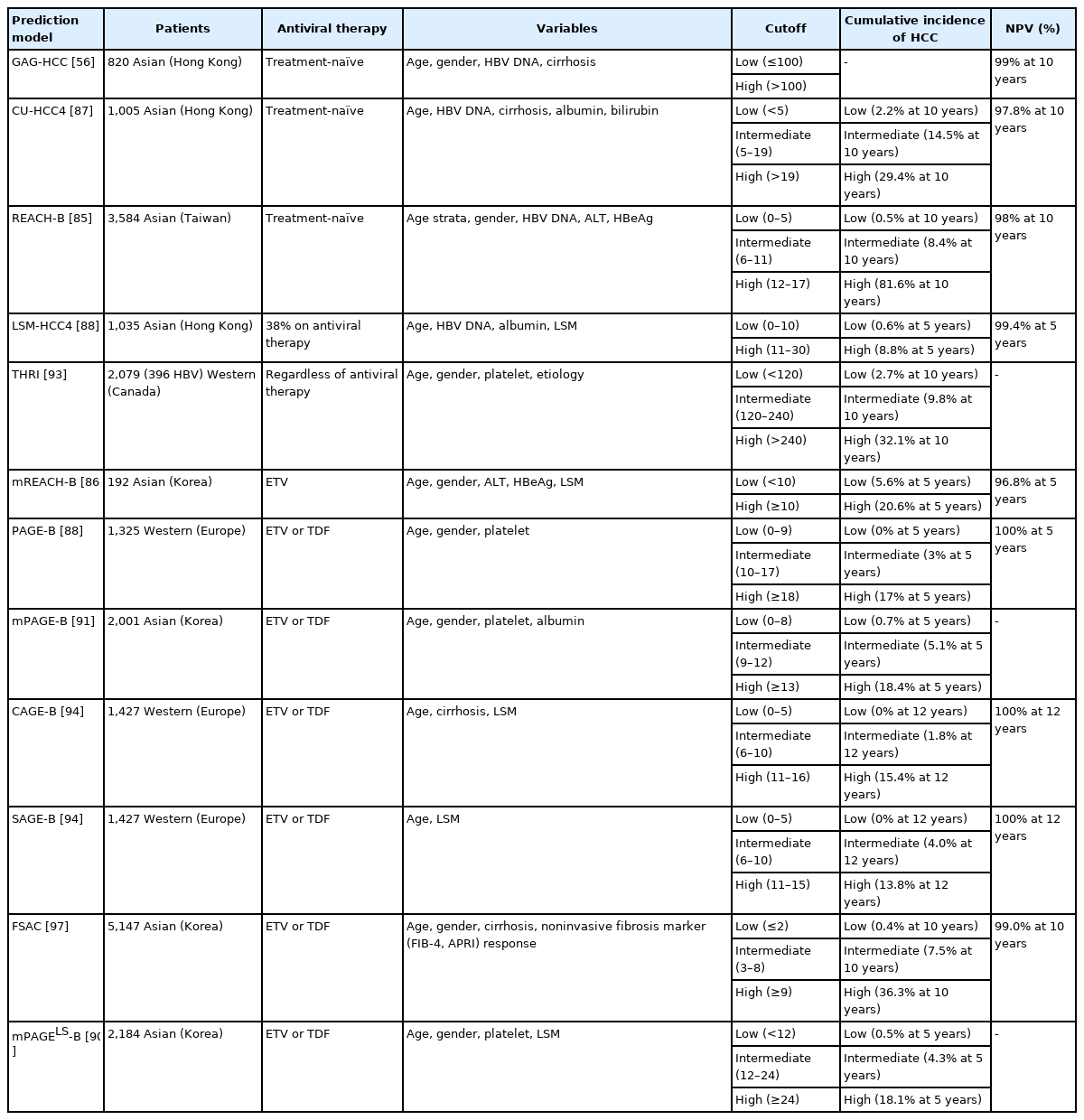
Multiple prognostic prediction models have been developed to estimate the risk of HCC in CHB patients (Table 5). The REACH-B (risk estimation for HCC in chronic hepatitis B) model, which consists of gender, age, serum ALT, HBeAg, and serum HBV DNA level, has been developed for HCC risk prediction in non-cirrhotic, treatment-naïve CHB patients. REACH-B model has been validated in Hong Kong and Korean cohorts of CHB patients including those with liver cirrhosis. Areas under the receiver operating characteristic curve for HCC prediction at 3 years, 5 years, and 10 years are 0.77–0.81 in those cohort [85]. Modified REACH-B (mREACH-B) model, which substituted serum HBV DNA for the liver stiffness value from the original REACH-B model, showed better outcomes in assessment of 3-year and 5-year HCC prediction in several prospective Korean studies [86,87]. Meanwhile, PAGE-B (platelets, age, gender, and hepatitis B) model, which was developed from Western studies [88], has been validated by several Korean retrospective studies [89,90]. Modified PAGE-B (adding serum albumin) was superior to the original PAGE-B in prediction of 5-year HCC risk in Korean CHB patients [90-92]. Although THRI (Toronto HCC risk index) has shown good predictive ability for HCC in patients with cirrhosis [93], it was not superior to mPAGE-B in Korean CHB patients [91]. Meanwhile, the CAGE-B (cirrhosis and age) and SAGE-B (stiffness and age) models, which were developed from Western studies [94], has been validated by several Korean retrospective studies [95,96]. A recently developed FSAC (fibrosis marker response, sex, age, cirrhosis) model that incorporates on-therapy changes in non-invasive fibrosis markers (fibrosis-4 [FIB-4] or aspartate aminotransferase-to-platelet ratio index [APRI]) has been validated in a separate cohort of Korean CHB patients [97].
Recently, deep learning or artificial intelligence-assisted prediction models were developed to predict the risk of HCC in patients with CHB on antiviral treatment, and these models demonstrated superior performance in risk stratification compared with previous risk scores [98,99].
Summary
1. The natural course of CHB is divided into five phases – immune-tolerant, HBeAg-positive immune-active, immuneinactive, HBeAg-negative immune-active, and HBsAg loss. Studies on the prognosis and treatment of grey zones that are not classified by clinical indicators, such as ALT and HBV DNA, are in progress.
2. Several models have recently been developed to predict the risk of HCC in CHB patients using clinical parameters, artificial intelligence, and deep-learning technology. There are increasing efforts to apply these models to clinical care in patients receiving antiviral treatment.
EMERGING MARKERS OF HBV INFECTION
HBV, the Dane particle, has a diameter of approximately 44 nm and consists of a double-layered capsid particle enclosing a circular, incomplete double-stranded DNA genome (3.2 kb in length). HBV has been classified into eight genotypes (A to H) by a divergence >8% in the entire genomic sequence. The distribution of HBV genotypes differs between regions and ethnicity. Genotype C, especially the C2 subgenotype, prevails predominantly among chronic HBV carriers in Korea [100,101]. Genotype C HBV infection is an independent risk factor for HCC development in addition to liver cirrhosis [102,103]. Patients infected with HBV genotype C tended to have persistently positive HBeAg or fluctuating HBeAg status.
The structure of HBV consists of an external surface protein and an internal core protein. The antigenicity to each protein is called HBsAg and HBcAg, respectively. There are four known genes encoded by the genome, called C, X, P, and S. Gene S codes for the HBsAg. The core protein (HBcAg) is coded for by gene C, and its start codon is preceded by an upstream in-frame AUG start codon from which the pre-core protein is produced. HBeAg is produced by proteolytic processing of the pre-core protein. HBsAg and HBcAg can be measured in peripheral blood because they circulate in serum. Otherwise, HBcAg is an intracellular antigen that is expressed in infected hepatocytes. It is not detectable in serum with available commercial assays. Antibodies for each antigen, such as anti-HBs, anti-HBc, and anti-HBe, can be detected by serological tests.
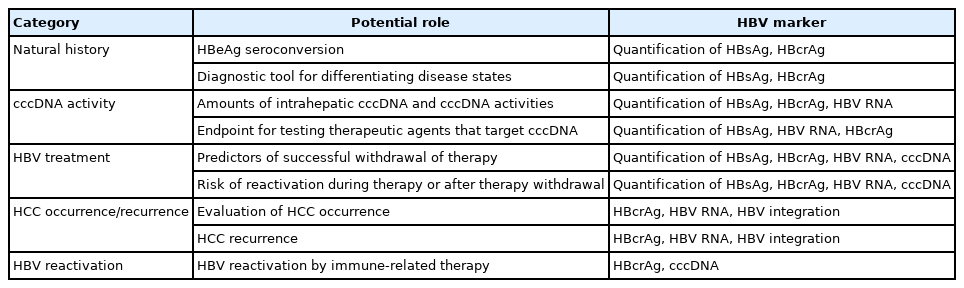
In addition to traditional tests above, novel virological markers have been developed to predict prognosis, treatment response, and off-treatment viral suppression in patients infected with HBV (Table 6, Fig. 2) [104].
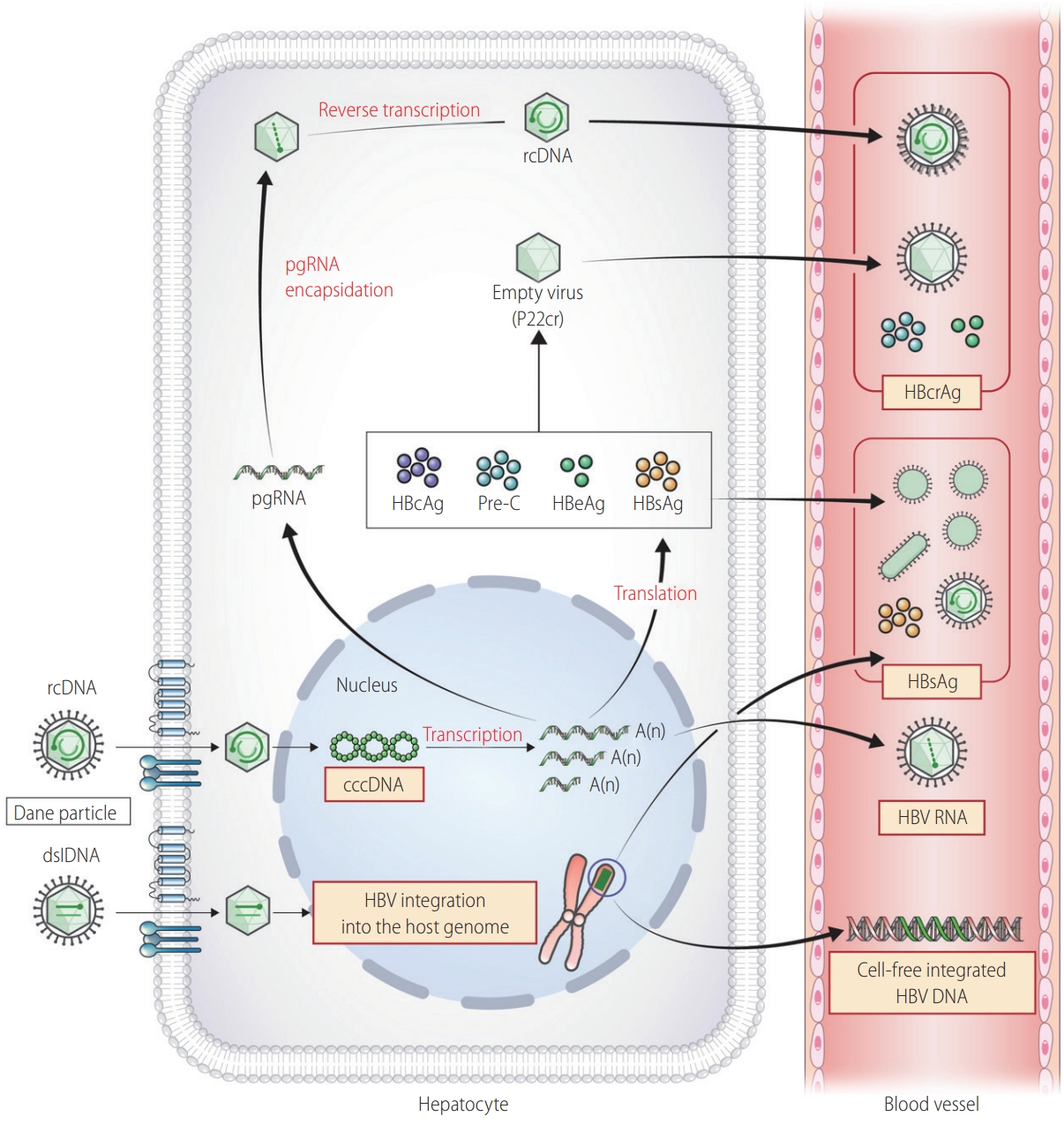
Emerging markers for chronic hepatitis B infection. rcDNA, relaxed circular DNA; pgRNA, pregenomic RNA; p22Cr, p22 core-related antigen; HBcAg, hepatitis B core antigen; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HBcrAg, hepatitis B core-related antigen; dslDNA, double stranded linear DNA; cccDNA, circular covalently closed DNA; HBV, hepatitis B virus.
Serum marker
Quantitation of HBsAg
The HBV encodes the three proteins of the HBsAg, which form the viral envelope, small (SHBsAg), middle (MHBsAg), and large (LHBsAg). These proteins are translated from two HBV subgenomic mRNA transcripts, the preS1 mRNA and the preS2/S mRNA. HBsAg is not only generated by transcription and translation of cccDNA, but also can be generated from HBV DNA episomally integrated into the host genome. HBsAg is assembled with core proteins and DNA polymerases during the viral replication process to form a complete virion (Dane particle). The surface proteins also generate non-infectious excess sub-viral particles, approximately 22 nm in diameter, with either a spherical or a long filamentous form. The quantitation of serum HBsAg is a method of detecting all three forms. There is a positive correlation between levels of serum HBsAg and intrahepatic cccDNA in patients with CHB.
Levels of HBsAg helps to distinguish inactive carriers from CHB patients. In HBeAg-negative patients, one-time measurements of serum HBV DNA <2,000 IU/mL and HBsAg <1,000 IU/mL suggest future inactive carriers [105,106]. In contrast, among HBeAg-negative patients with lower viral load (HBV DNA <2,000 IU/mL), HCC risk is higher in those with a high HBsAg titer (>1,000 IU/mL) than in those with a low HBsAg titer [107]. Levels of HBsAg can predict response during pegylated interferon (peginterferon) therapy in HBeAg-positive patients, possibly providing a guide to stopping treatment earlier [108]. For nucleos(t)ide analogue (NA) treatment, levels of HBsAg has emerged as a valuable tool in identifying patients who will maintain or attain inactive status or develop HBsAg loss if they discontinue long-term NA therapy [109-111].
Hepatitis B core-related antigen (HBcrAg)
HBcrAg is a composite biomarker incorporating several viral antigens expressed from the pre-core/core gene: the HBcAg, HBeAg, and p22 core-related antigen (p22Cr) [112]. Although it is not yet commonly used in Korea, HBcrAg can be easily detected using a chemiluminescent immunoassay kit. Significant positive correlations between serum HBcrAg level and HBV DNA level, as well as amount of intrahepatic cccDNA, have been observed in several Asian and European studies [113]. Serum HBcrAg level varies significantly among the phases of HBV infection. HBcrAg is, therefore, also a good virologic marker to differentiate HBeAg-negative CHB patients (active disease) from HBeAg-negative chronic HBV infection (inactive disease) [114]. Level of HBcrAg can potentially predict the possibility of achieving partial cure in patients on antiviral treatment, as defined by a sustained off-therapy virological control. In the study of 130 Hong Kong patients with undetectable serum HBV DNA during NA, HBcrAg was detectable in 101 (78%) samples [115]. After eight years of NA treatment, 21.3% of patients achieved serum HBcrAg <3 log10 U/mL [116]. Based on these findings, monitoring of HBcrAg and HBsAg quantification is recommended by the Japan Society of Hepatology guidelines to identify patients who can discontinue NA [117]. Furthermore, HBcrAg can be an aid for clinicians in identifying patients with a higher risk of HCC development or post-treatment recurrence [118,119]. Recently, the HBcrAg level has been the most emerging noninvasive predictor reflecting intrahepatic viral replication.
HBV RNA
Serum HBV RNA represents partially reverse-transcribed, encapsidated pregenomic RNA (pgRNA) in virus-like particles. Given that pgRNA is transcribed directly from cccDNA, level of serum HBV RNA can potentially serve as a surrogate marker for transcriptionally active cccDNA [120]. Serum HBV RNA may be an indicator for predicting off-treatment response in patients with suppressed HBV under NA treatment [121,122], although it has limited additional value in differentiating CHB phases [123]. Measurement of HBV RNA could be used to determine cccDNA activity, especially with therapies in development that aim for functional cure [119,124]. Levels of HBV RNA is also associated with increased HCC risk and post-resection recurrence in NA-treated patients [125,126]. Before a widespread clinical application of serum HBV RNA for CHB can be accepted and applied, the methodology for detecting and quantifying serum HBV RNA should be standardized.
Intracellular marker
HBV cccDNA
In the nucleus of hepatocytes, cccDNA is maintained as a stable mini-chromosome and acts as the template for pgRNA transcription and viral protein production [127]. The existence of intrahepatic cccDNA is the molecular basis of disease chronicity and represents a major barrier to HBV eradication. cccDNA is responsible for persistence of the virus in the liver even after HBsAg loss and seroconversion. Occult HBV infection is a state in which HBsAg is not detected in serum, but HBV DNA is present in liver tissue or blood, which is believed to be due to cccDNA remaining in the nucleus. cccDNA also causes hepatitis B reactivation in patients receiving immunosuppressants or chemotherapy [128]. In terms of HBV treatment, current widely used NAs can block the transcription process of RNA production from cccDNA but not remove cccDNA itself. Therefore, cccDNA acts as a major cause of HBV reactivation after stopping the NA, and the development of drugs that can remove cccDNA itself is essential to cure hepatitis B [129]. In order to quantify cccDNA in the liver, invasive liver biopsy is required, making it difficult to use as a marker in clinical settings.
HBV integration into the host genome
HBV can integrate in human DNA and promote hepatocarcinogenesis by insertional mutagenesis, increased genomic instability, or expression of viral oncoproteins such as the protein HBx [130,131]. The application of next-generation sequencing led to characterization of HBV integration events. Recent studies reported that, among patients with primary liver cancer, a higher number of HBV integration events was associated with worse outcome [130,132-134]. In addition, cell-free HBV-integrated tumor DNA was shown to be a circulating biomarker for detecting tumor load in a majority of patients with HBV-related HCC and aided in monitoring residual tumor and recurrence clonality after tumor resection [135,136].
HBV pgRNA
Among HBV mRNAs, 3.5-kb preC and pgRNA originate from HBV cccDNA, and pgRNA is reverse transcribed to enable the synthesis of HBV DNA. Intrahepatic pgRNA is considered a marker of active HBV replication. RNA interference (RNAi) therapeutics such as siRNA and anti-sense molecules targeting pgRNA are being developed. However, the role of intrahepatic pgRNA as a marker for treatment response has recently been replaced by serum HBV RNA measurements.
Summary
1. Serum HBsAg, HBcrAg, and HBV RNA measurements can be valuable indicators in stratifying CHB phases, determining suitability for NA cessation, and predicting off-treatment response.
2. Intracellular markers of HBV such as cccDNA and pgRNA can be effective targets for drug development for HBV cure.
TREATMENT INDICATION
Active HBV replication is associated with increased risk of liver damage, progression of liver disease, and liver-related complications [22]. Antiviral therapy can effectively inhibit replication of the virus [137]. Inhibition of HBV replication by antiviral therapy can improve hepatic inflammation, normalize serum ALT level, improve liver fibrosis, reduce the incidence of HCC, and decrease liver-related death [138].
However, currently available antiviral therapies cannot eradicate or eliminate the virus. Furthermore, the efficacy and side effects of the same drug can vary depending on the clinical situation [137]. Therefore, benefits and risks of antiviral therapy should be carefully evaluated on an individual basis. The following three factors are fundamental components that should be taken into consideration when deciding antiviral therapy: 1) the severity of liver disease, 2) the degree of HBV replication, and 3) the presence of liver injury (Fig. 3). The severity of liver disease can be categorized into chronic hepatitis, compensated cirrhosis, and decompensated cirrhosis according to the degree of liver fibrosis. Severity of liver fibrosis can be evaluated by liver biopsy or non-invasive methods using serum markers (e.g., APRI, FIB-4 index, M2BPGi) [118,139] or transient elastography (TE) using Fibroscan® (Echosense, Paris, France) [140,141]. In general, greater than F2 fibrosis in liver biopsy is considered significant [142], and the diagnostic cut-off for F2 is 7.8 kPa in Fibroscan® in a meta-analysis from Korea [140]. The degree of HBV replication can be assessed by measuring serum HBV DNA level. The presence of liver injury can be estimated using serum ALT level or can be assessed by a liver biopsy. When it is accompanied by inflammation greater than grade A2–A3 in liver biopsy, it is defined as moderate or more severe inflammation [142].
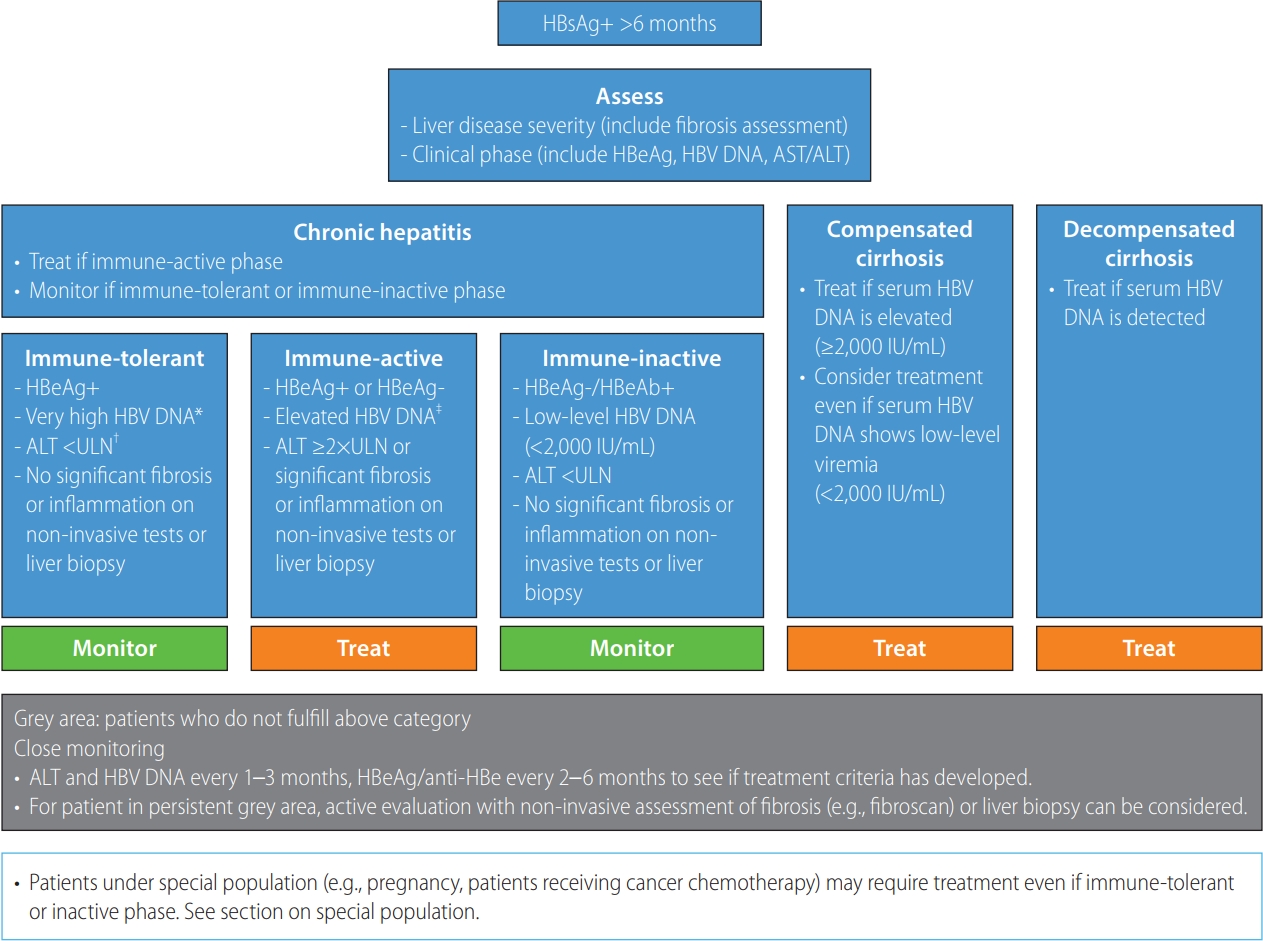
Algorithm for management of chronic hepatitis B virus infection. HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; AST, aspartate aminotransferase; ALT, alanine aminotransferase; anti-HBe, hepatitis B e antibody. *Serum HBV DNA ≥107 IU/mL. †An upper limit of normal (ULN) for ALT of 34 IU/L for males and 30 IU/L for females. ‡Serum HBV DNA ≥20,000 IU/mL for HBeAg-positive patients and HBV DNA ≥2,000 IU/mL for HBeAg-negative patients.
CHB, immune-tolerant phase
The immune-tolerant phase is characterized by HBeAg positivity, very high serum HBV DNA level (usually ≥107 IU/mL), and persistently normal serum ALT level. In this phase, long-term prognosis is excellent without antiviral therapy [143-145]. In a long-term observation of CHB immune-tolerant patients in Korea, the incidence of HCC at 10 years was very low (1.7–2.7%) and not higher than that of patients in the immune-active phase who received antiviral treatment [5,146,147]. To verify the immune-tolerant phase, a liver biopsy is necessary and will show no or mild inflammation without fibrosis. However, liver biopsy is an invasive procedure with potential complications that limit its widespread use and repetitive testing in clinical practice. Hence, in real-life clinical practice, a combination of clinical findings (HBeAg positivity, high serum HBV DNA level, normal ALT level, and no evidence of cirrhosis) is typically used to define the immune-tolerant phase without liver biopsy. However, caution should be exercised considering the results of a recent study suggesting that HCC and liver cirrhosis-related complications occur in a considerable number of such patients during long-term follow-up [7]. In the study, the rate of HCC at 10 years was high (12.7%) compared to another study, although enrolled patients showed HBeAg positivity, HBV DNA (>20,000 IU/mL), normal ALT level, and no evidence of cirrhosis [7].
In recent studies, older age, male, relatively low serum HBV DNA level [7,148], high liver stiffness value [147,149], and normal but high-normal ALT level were associated with HCC development or liver-related complications among patients presumed to be in the immune-tolerant phase by combinations of clinical findings without a liver biopsy [7].
The immune-tolerant phase is usually characterized by little or no necroinflammation without liver fibrosis, but significant fibrosis as seen using non-invasive serum fibrosis markers (e.g., APRI, FIB-4, M2BPGi) or TE (Fibroscan®) suggests that antiviral treatment can be considered. The immune-tolerant phase is usually observed in young adults and is not common in elderly patients as immune reaction against HBV onsets with age. Indeed, elderly patients in the presumed immune-tolerant phase showed higher possibility of significant fibrosis (≥F2) or necroinflammation (≥A2) in liver biopsy [150], and increased risks of HCC development or liver-related complications [7,148]. Therefore, even when all the other clinical findings suggest the immune-tolerant phase, a liver biopsy can be considered to verify the immune-tolerant phase in older adults. An age cut-off for liver biopsy consideration was suggested to be 30–40 years [144,151], but evidence to support this approach is limited. Also, non-invasive serum fibrosis markers (e.g., APRI, FIB-4, M2BPGi) or TE (Fibroscan®) can be used to evaluate the degree of hepatic fibrosis and is helpful in distinguishing patients with genuine immune tolerance. Among CHB patients without clinical cirrhosis, those with a higher liver stiffness value (≥13 kPa) showed a higher rate of HCC than those with a lower value (<13 kPa) [149]. In contrast, the cumulative probability of HCC at 5 years was negligible in the immune-tolerant group stringently defined by a low FIB-4 index (<1.45) [147].
The immune-tolerant phase is also characterized by very high level of HBV DNA, as there is little or minimal immune response to the virus [144,151]. In one study, among patients with HBeAg positivity and normal ALT level, relatively low serum HBV DNA level (<107 IU/mL) was associated with a higher risk of HCC and death compared to those with very high serum HBV DNA level (≥107 IU/mL) [7,148]. Relatively low serum HBV DNA level indicates that the immune response has already begun to suppress the virus. In a recent study about treatment-naïve CHB patients with ALT <2 times the ULN, HBV DNA >106 IU/mL, HBeAg positivity, and HCC risk was highest with baseline HBV DNA level of 106–7 IU/mL compared to HBV DNA level greater than 107 IU/mL [152].
ALT is a good indicator of liver necroinflammation, so patients in the immune-tolerant phase show persistently normal ALT level, as there is no or little liver necroinflammation. Patients with slightly elevated ALT level are more likely to have fibrosis and necroinflammation on a liver biopsy and have a higher risk of complications during follow-up [148,150]. Therefore, ALT at the borderline of or slightly higher than ULN is a sign that a patient is not genuinely in the immune-tolerant phase. However, careful interpretation is needed in defining normal or elevated ALT level. There is controversy about what constitutes healthy, normal ALT level. Elevation of ALT can be caused by obesity and other conditions not related to HBV. Recently, the cut-off level for ALT associated with increased liver-related mortality among Korean chronic HBV-infected patients was reported to be 34 IU/L for men and 30 IU/L for women [153]. Therefore, the present guidelines recommend using these values to define normal ALT level.
The efficacy of currently available antiviral regimens is limited for patients in the immune-tolerant phase. Antiviral treatment using NAs resulted in a poor antiviral response rate and a low HBeAg seroclearance rate [154]. Furthermore, when NA treatment was discontinued for those who started oral NA therapy at the immune-tolerant phase, all patients showed a rebound of serum HBV DNA level above 2,000 IU/mL, 70% showed an elevation of ALT level, and 55% had to re-start NA therapy [155]. Long-term treatment might be necessary, and treatment discontinuation can be difficult. However, in one study from Korea that compared 87 NAtreated immune-tolerant CHB patients to 397 monitored immunetolerant patients as a control group, increased risk of HCC and cirrhosis was observed in the control group despite favorable baseline liver function [156]. In a recent Korean analysis about immune-tolerant phase patients based on several studies, starting antiviral therapy in this phase is cost-effective compared with delaying treatment until the active phase in CHB patients, especially considering increasing HCC risk, decreasing drug costs, and consideration of productivity loss [157]. This finding suggests that some patients who are presumed to be in the immune-tolerant phase will develop complications during follow-up, and that antiviral treatment can decrease this risk. Further studies are needed to identify appropriate antiviral treatment indications in patients in the immune-tolerant phase.
[Recommendations]
1. CHB patients in the immune-tolerant phase, as defined by HBeAg positivity, very high serum HBV DNA level (≥107 IU/mL), persistently normal ALT level, and no inflammation or fibrosis on liver biopsy, should be monitored without antiviral therapy (B1).
2. Evaluation of the degree of liver fibrosis using non-invasive fibrosis tests or liver biopsy among presumed immunetolerant phase patients with normal ALT level is suggested if the patient’s age is ≥30–40 years, serum HBV DNA level is <107 IU/mL, or ALT level is approaching the borderline of ULN, and antiviral therapy can be considered for patients with significant liver fibrosis (B2).
HBeAg-positive and HBeAg-negative CHB, immune-active phase
The immune-active phase is characterized by active replication of HBV and moderate or severe necroinflammation with or without fibrosis. A systematic review and meta-analysis of 15 randomized controlled trials and 44 observational studies showed that antiviral treatment in the immune-active phase reduced the risk of cirrhosis, hepatic decompensation, and HCC [138]. Therefore, patients in the immune-active phase are indicated for antiviral treatment. Nevertheless, careful attention to HCC development is needed as antiviral treatment cannot completely eliminate the risk of HCC [158]. A recent study from Korea reported a marked reduction in liver disease mortality by widespread use of antiviral treatments against HBV but paradoxical increased burden of liver cancer [159].
Active replication of HBV can be confirmed by serum HBV DNA measurement using polymerase chain reaction. Detection of HBV DNA in the serum indicates active replication of the virus. However, the lower limit of detection is different among HBV DNA assays. Moreover, many patients with low-level viremia (serum HBV DNA level <2,000 IU/mL) show normal ALT level, little or no necroinflammation or fibrosis on liver biopsy, and favorable outcomes without antiviral therapy [68]. Hence, not all patients with detectable serum HBV DNA, but patients with serum HBV DNA level ≥2,000–20,000 IU/mL for HBeAg-positive patients and serum HBV DNA level ≥2,000 IU/mL for HBeAg-negative patients are considered for antiviral therapy [56,68,70].
Serum ALT is a convenient indicator of necroinflammation of the liver and can be easily used in clinical practice [160]. Elevation of ALT suggests hepatocellular injury and requires assessment and evaluation. However, the degree of ALT elevation does not always correlate with necroinflammation of the liver and can be affected by body mass index and gender [161,162], alcohol use, drug use, fatty liver, and other causes unrelated to HBV [162,163]. A normal ALT level does not exclude significant liver disease [164]. Hence, the use of ALT as a criterion for treatment initiation requires consideration of the threshold of elevation. If the ALT level is elevated more than ≥2 times the ULN, antiviral treatment for HBV is recommended unless the increase is due to other causes [144,151]. When ALT is elevated above but <2 times the ULN, controversy exists as to whether these patients require antiviral treatment [144,151]. Patients with serum ALT elevated above but <2 times the ULN have increased risk of liver cirrhosis and HCC compared to patients with serum ALT within the normal range [165,166]. Yet, “normal” ALT level varies among studies and by ethnicity [162,167]. The specific ALT levels used in clinical trials to initiate antiviral therapy also differ [168-173]. Therefore, sufficient data are not available to judge whether it is necessary to start antiviral treatment in patients with serum ALT elevated above but <2 times the ULN. In this case, trends in serum ALT and HBV DNA levels should be closely monitored to identify possible causes and to verify whether treatment for such patients should be initiated (Fig. 3). If a patient shows persistently elevated ALT level <2 times the ULN, the degree of fibrosis can be further investigated by non-invasive fibrosis tests or by liver biopsy to verify whether patients require antiviral treatment due to significant fibrosis.
Histological assessment of the liver, liver biopsy, is a cornerstone in the evaluation of hepatic necroinflammation and fibrosis[174]. Findings of moderate to severe necroinflammation or significant fibrosis (≥F2) indicate that antiviral treatment for HBV is needed [137]. However, a liver biopsy is an invasive procedure requiring special resources that limit widespread clinical use. Serum fibrosis biomarkers or TE (Fibroscan®) of the liver are alternatives that can be used to estimate degree of fibrosis [175]. These non-invasive biomarkers for liver fibrosis are less accurate than liver biopsy but can be used to rule in or rule out patients with significant fibrosis. HBeAg, HBV DNA concentration, and ALT level have been traditionally used to determine starting antiviral therapy, and liver biopsy was used in a case of difficult decision for antiviral therapy [176]. Recently, treatment initiation based on liver disease severity as assessed by non-invasive tests (e.g., Fibroscan®) has been suggested [175]. However, more evidence is needed for cut-off levels of non-invasive tests to support treatment initiation.
Among HBeAg-positive CHB patients, spontaneous HBeAg seroconversion has been reported for those experiencing increase of ALT level with HBV DNA elevation. Hence, 3–6 months of observation without antiviral treatment can be considered if spontaneous HBeAg seroconversion is expected [176]. However, biochemical deterioration leading to liver failure is of concern. A prospective cohort study of 90 patients from Korea with HBeAg-positive CHB who were monitored without antiviral therapy showed a very low rate of spontaneous HBeAg seroconversion (1.1%), while there was frequent biochemical deterioration and one case of liver transplantation due to liver failure [177]. Therefore, when expecting HBeAg seroconversion, the risk of acute decompensation leading to liver failure warrants careful attention. Another report from Korea showed that spontaneous HBeAg seroconversion can be expected for patients with non-vertical transmission and low serum HBV DNA level [178].
CHB patients can present with severe acute exacerbation, characterized by elevated HBV DNA level, serum ALT level 5–10 times greater than ULN, jaundice, coagulopathy, ascites, and/or hepatic encephalopathy. They can also be classified as having acute-on-chronic liver failure (ACLF) when they present with symptoms and signs of liver failure [179]. Severe acute exacerbation can occur spontaneously [180], by drug-resistant HBV during antiviral therapy [181], with cessation of antiviral therapy [182], or with anticancer chemotherapy [183]. NA therapy reduces mortality in patients with severe reactivation of CHB presenting as ACLF [184]. Therefore, immediate antiviral treatment is recommended for CHB patients with severe acute exacerbation or ACLF [176]. Some studies have reported a higher mortality rate among entecavir-treated patients than lamivudine-treated patients [185,186], but a meta-analysis of three prospective and eight retrospective studies showed similar effects on the mortality rate between entecavir and lamivudine treatment, with a more favorable long-term outcome in entecavir [179]. However, antiviral treatment cannot fully prevent progression to liver failure, which can lead to mortality in the case of high Model for End-stage Liver Disease (MELD) score, moderate to severe ascites, and/or aggravation of hepatic encephalopathy [187-189], Emergent liver transplantation should be considered and prepared. Steroid or plasma exchange has been suggested in cases of severe acute exacerbation and ACLF, but data are currently limited to a small number of cases [190,191].
Some HBeAg-negative CHB patients show normal or mildly elevated ALT level despite elevated HBV DNA level (>2,000 IU/mL). Some patients progress to the immune-inactive phase spontaneously—especially patients with low HBsAg level and low serum HBV DNA level [192]. In a recent study about patients with HBeAg negativity and replicative HBV DNA ≥2,000 IU/mL, according to ALT level (persistently normal ALT, ALT 1–2 times the ULN, ALT >2 times the ULN), untreated patients with ALT 1–2 times the ULN had higher risks of HCC and death/transplantation than antiviral-treated patients with ALT >2 times the ULN [54]. HBeAg-negative patients are those who have experienced the prior immune-active phase, and there is possibility that various degrees of fibrosis remain in these patients. For those with advanced fibrosis, antiviral treatment can be considered for those with elevated HBV DNA level regardless of ALT level [138,151]. Hence, HBeAg-negative CHB patients showing elevated HBV DNA level (>2,000 IU/mL) but normal or mildly elevated ALT level require careful evaluation of their degree of fibrosis to decide if they should undergo antiviral treatment or monitoring.
[Recommendations]
1. Antiviral therapy is recommended in HBeAg-positive CHB patients with HBV DNA ≥20,000 IU/mL and in HBeAgnegative CHB patients with HBV DNA ≥2,000 IU/mL if serum ALT level is ≥2 times the ULN (A1). In cases where ALT is 1–2 times the ULN, close ALT monitoring or liver biopsy should be considered. Antiviral therapy is recommended if liver biopsy reveals moderate to severe necroinflammation or significant fibrosis (≥F2) (A1), which can be assessed by non-invasive fibrosis tests (B1).
2. In patients with HBeAg-positive or HBeAg-negative CHB, prompt antiviral therapy should be initiated in cases of acute exacerbation, with elevation of ALT ≥5–10 times the ULN and signs of liver failure such as jaundice, prothrombin time prolongation, ascites, or hepatic encephalopathy (A1).
3. In HBeAg-negative CHB patients with HBV DNA ≥2,000 IU/mL and normal ALT level, follow-up can be considered. Otherwise, liver biopsy or non-invasive fibrosis tests can be considered for assessment of the degree of necroinflammation and/or fibrosis to determine whether treatment is needed (B2).
CHB, immune-inactive phase
The immune-inactive phase is characterized by HBeAg-negative, anti-HBe-positive, persistently normal ALT level, and undetectable or low (<2,000 IU/mL) serum HBV DNA level. In this phase, long-term outcome without antiviral treatment is good for those without advanced fibrosis [68]. In contrast, risk of HCC is not low for these patients with high FIB-4 or APRI index suggesting advanced liver fibrosis [52,54,193,194]. The immune-inactive phase is a dynamic phase that can reactivate to an immune-active phase [14]. Hence, patients in the immune-inactive phase require careful assessment of the degree of fibrosis and close monitoring of serum ALT and HBV DNA levels to verify whether they remain in the immune-inactive phase.
HBsAg loss or seroclearance is observed in 1–2% of patients per year in the immune-inactive phase [39,41]. HBsAg seroclearance is considered a surrogate endpoint for a functional cure of CHB. Hence, several studies investigated whether antiviral therapy in the immune-inactive phase can further induce HBsAg seroclearance. In a study about immune-inactive phase patients with HBsAg <1,000 IU/mL, HBsAg loss was achieved in 44.7% of 102 patients treated with peginterferon or peginterferon/adefovir compared to only 2.4% of 42 patients without treatment over 96 weeks. This study suggested that antiviral therapy in immune-inactive phase patients with low HBsAg titer could be considered for HBsAg loss [195]. However, in a randomized prospective controlled study for 48 weeks, when 151 immune-inactive phase patients irrespective of HBsAg titer were randomized into two groups, the treatment group with peginterferon/adefovir or peginterferon/tenofovir (DF or AF) and the no-treatment group, the rate of HBsAg loss was not different between the two groups at 72 weeks (4% vs. 0%, respectively) [196]. This suggests that the benefit of antiviral therapy is limited in immune-inactive phase patients, who have low risk for HCC or liver-related complications during follow-up. The clinical benefit of inducing HBsAg loss by antiviral treatment in the immune-inactive phase, in terms of achieving treatment goals for CHB (improving overall survival or preventing development of HCC) has not yet been demonstrated and requires further investigation.
[Recommendations]
1. Antiviral treatment is not indicated in CHB patients in the immune-inactive phase, determined by serum HBV DNA <2,000 IU/mL, a normal ALT level, and no evidence of advanced liver fibrosis (B1).
2. Antiviral treatment can be considered in CHB patients in the immune-inactive phase with significant liver fibrosis suggested by liver biopsy or non-invasive fibrosis tests, even if the serum HBV DNA is less than 2,000 IU/mL (B2).
Compensated cirrhosis
Liver cirrhosis could be diagnosed by liver biopsy. Since liver biopsy is limited in real practice, clinical diagnosis of cirrhosis is usually assigned when an image study (computed tomography, abdominal ultrasonography, and magnetic resonance imaging) shows nodular liver surface, splenomegaly, or presence of intraabdominal collaterals suggesting portal hypertension or when endoscopy reveals esophageal or gastric varix or clinical symptoms of cirrhosis [197]. In addition to imaging studies, laboratory findings such as serum albumin, bilirubin, prothrombin time, and platelets can be helpful in diagnosis of liver cirrhosis.
Antiviral treatment for compensated cirrhosis patients can decrease the risk of HCC and liver-related complications [138] and improve liver fibrosis [198,199]. Serum ALT level might not be elevated in patients with cirrhosis, and the risk of complication is high even for those with normal ALT level [200]. Hence, cirrhotic patients with active HBV replication require antiviral treatment regardless of ALT level. For cirrhotic patients, the risk of HCC decreases but remains even after achieving a virological response to antiviral therapy [201], requiring HCC surveillance.
For compensated cirrhosis patients, those with elevated HBV DNA level (≥2,000 IU/mL) are indicated for antiviral therapy. For patients with detectable but low-level viremia (<2,000 IU/mL), recent European Association for the Study of the Liver and American Association for the Study of Liver Diseases guidelines recommend antiviral therapy [144,151]. An observational cohort study from Korea reported that 33% of compensated cirrhosis patients with low-level viremia experienced HBV DNA elevation ≥2,000 IU/mL during follow-up, and this was associated with increased risk for HCC [202]. Furthermore, HCC risk was higher for patients who remained at low-level viremia compared to those with undetectable HBV DNA level, antiviral treatment was inversely associated with HCC risk in this group, and antiviral treatment could decrease the risk of reactivation of HBV and have survival benefit in patients with low-level viremia even after HCC development [202,203]. For compensated cirrhosis patients with low-level viremia, prompt antiviral treatment has the advantage of preventing HBV DNA elevation during follow-up and can decrease the risk of complications, as shown in another observational study from Korea. In that study, the cumulative incidence rate of HCC among cirrhotic patients with low-level viremia was 13.9% in 10 years, and they showed higher risk of death and liver-related complications than did treated cirrhotic patients [193,204]. These data support prompt antiviral therapy for compensated cirrhosis with low-level viremia. However, in a recent study from Korea, the incidence rate of HCC of compensated cirrhotic patients was not different between those with HBV DNA <2,000 IU/mL without antiviral treatment and those with complete virological response to antiviral treatment [205]. However, until now, there have not been any randomized controlled trials that can assess the benefits and risks of prompt antiviral therapy for compensated cirrhosis patients showing lowlevel viremia.
[Recommendations]
1. In patients with compensated cirrhosis, antiviral therapy should be initiated regardless of ALT level if serum HBV DNA level is ≥2,000 IU/mL (A1).
2. Antiviral therapy can be initiated in compensated cirrhosis patients regardless of ALT level, even in those with detectable but low-level viremia (<2,000 IU/mL) (B1).
Decompensated cirrhosis
Decompensated cirrhosis includes cases with ascites, variceal bleeding, hepatic encephalopathy, or jaundice [197]. Patients with decompensated cirrhosis might be managed in an institution that can respond appropriately to complications and are candidates for liver transplantation. Antiviral therapy modifies the natural history of decompensated cirrhosis, improves liver function, decreases the need for liver transplantation, and improves survival [206,207]. However, even if antiviral therapy is administered, it takes time to produce a virological response and recover clinically. Some patients with severely impaired liver function do not recover despite antiviral therapy, and liver transplantation should be considered for such cases [208]. Patients with decompensated cirrhosis are prone to liver failure with HBV reactivation, which requires prompt antiviral therapy when serum HBV DNA is detectable, regardless of its serum level. Administration of interferon is contraindicated because it can cause serious side effects including liver failure even with small doses [209].
[Recommendations]
1. In patients with decompensated cirrhosis, NAs should be initiated if serum HBV DNA is detected regardless of ALT level. Liver transplantation should also be considered (A1).
NEW DRUGS FOR A FUNCTIONAL CURE
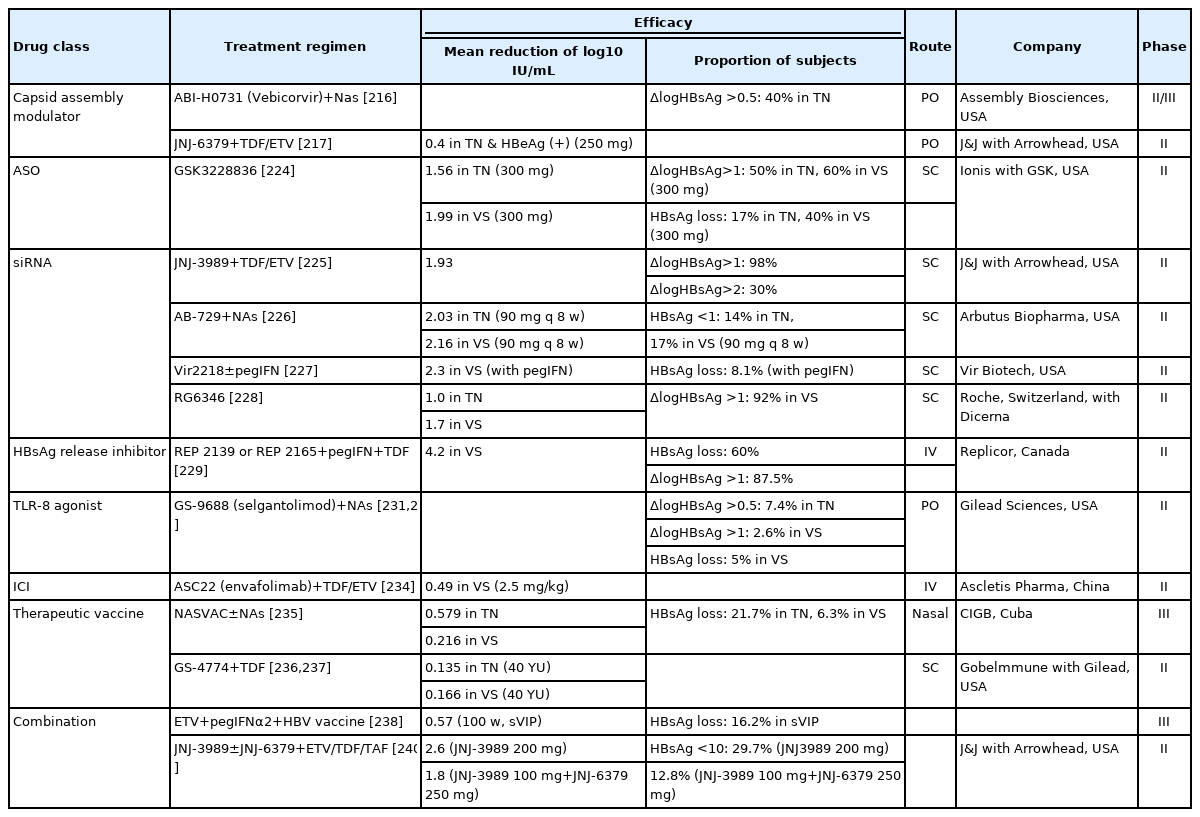
HBsAg seroclearance is the desired endpoint of functional cure for CHB. However, it cannot be fully achieved with conventional treatment including interferon or NAs [210]. Thus, many new drugs are being developed and investigated for functional cure of CHB, some of which have proceeded to phase 2 trials (Table 7). These drugs can be classified into two types based on mechanism of action: (1) drugs that directly interfere with the HBV life cycle and (2) drugs that strengthen the host immune response to HBV infection (Fig. 4). However, as safe and effective NAs are already available, the success of any newly developed drug will warrant significant reduction in serum HBsAg titer and few side effects.

The mechanisms of novel antiviral agents for the functional cure. NK, natural killer; TLR, toll-like receptor; ICI, immune checkpoint inhibitor; HBV, hepatitis B virus; NTCP, sodium taurocholate co-transporting polypeptide; SVP, subviral particle; HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B e antigen; HBc, hepatitis B core; HBx, hepatitis B virus X protein; cccDNA, covalently closed circular DNA; ASO, antisense oligonucleotide; siRNA, small interfering RNA.
Virus-targeting agents
Virus-targeting agents focus on viral genomes or proteins crucial for the viral life cycle to disrupt its replication and infection. These agents for treatment of CHB decrease titers of cccDNA or HBsAg, and include core protein inhibitors, RNA targeted therapeutics, HBsAg release inhibitors, and gene-editing agents.
Capsid assembly modulator
The HBV core protein aids in capsid assembly and imports pgRNAs and polymerases into the capsid for reverse transcription. Then, enveloped HBV DNA is transmitted to infect other hepatocytes or reenter the nucleus for viral cccDNA replenishment. Thus, inhibition of encapsidation could induce reduction of cccDNA expression [211,212].
NVR 3-778, ABI-H0731, and JNJ-6379 are core protein inhibitors developed as oral drugs. Clinical studies of these drugs have reported a significant reduction in serum HBV DNA but a minor reduction in serum HBsAg titer. Therefore, use of core protein inhibitors alone might be inadequate for functional cure of CHB. Thus, combination therapies with interferon or NAs were tested, but the decrease in serum HBsAg titer remained nonsignificant compared to that in the control group [213-217].
A recent study from Korea demonstrated that ciclopirox, a synthetic antifungal agent, strongly inhibits viral replication by blocking the HBV capsid assembly. When combined with NAs, ciclopirox significantly reduced the serum HBV DNA level and HBsAg titer in in vitro and in vivo studies [218,219]. Preclinical studies for the same are currently underway.
RNA targeted therapeutics
Viral RNA forms the backbone of viral antigens and proteins. RNAi inhibits translation of viral transcripts to prevent its replication and HBsAg production, restore HBV-specific immune response, and potentially lead to a functional cure [220]. Currently, two therapeutics are being used for RNAi-based therapy: antisense oligonucleotides (ASO) and small interfering RNAs (siRNAs). ASOs are 15–20-nucleotide-long single-stranded DNA oligomers, which binds to its complementary site on the target viral RNA to form a DNA-RNA duplex. The viral RNA in the DNA-RNA duplex is then cleaved by ribonuclease H, inhibiting the expression of the corresponding gene. On the other hand, siRNAs are 20–25 nucleotide-long double-stranded RNA molecules. The siRNA guide strand directs the RNA-induced silencing complex (RISC) to the complementary target RNA, which is then cleaved by the Argonaute protein present in the RISC [221,222]. Lipid nanoparticles or N-acetylgalactosamine (GalNac/NAG)-linked particles are used to deliver these agents to hepatocytes [222,223].
The main concerns regarding these agents include the risk of off-target toxicity, the potential toxicity of the delivery vehicle, and the risk of post-treatment reactivation by remaining cccDNA [219]. In addition, ALT flare should be monitored during the course of the treatment [224].
In the ASO, a phase 2 clinical study was conducted for GSK3228836, and both treatment-naïve and previously NA-treated groups each receiving 300 mg six times showed a mean reduction of more than 1.5 log10 IU/mL in serum HBsAg titer [224]. Several siRNA drugs are in phase 2 clinical trials, and interim analyses were presented at an international conference. With NA co-administration, JNJ-3989 induced a decrease in HBsAg greater than 1.0 log10 IU/mL in 98% of the participants and an average decrease of 1.93 log10 IU/mL [225]. Similar reduction in HBsAg titer was shown with AB-729, Vir-2218, and RG6346 [226-228].
HBsAg release inhibitor
HBsAg release inhibitors suppress the assembly and secretion of incomplete HBV particles (subviral particles, SVPs) to reduce serum HBsAg titer and mitigate immune exhaustion in the host, enhancing the HBV-specific immune response. Most of these drugs are nucleic acid polymers, of which REP 2139 and REP 2165 have completed phase 2 clinical trials. After 48 weeks of triple combination therapy with peginterferon and tenofovir disoproxil fumarate (tenofovir DF) in patients with CHB, HBsAg loss was observed in 60% of the patients. Among them, 58.3% maintained HBsAg loss for 48 weeks after discontinuation of REP 2139 or REP 2165 [229]. In addition, both these drugs showed a significant therapeutic effect in the treatment of HBV and hepatitis D virus (HDV) co-infection, supporting the potential of HBsAg release inhibitors for the effective treatment of HDV infection [230].
Immune modulators
One of the viral mechanisms driving CHB pathogenesis is to overwhelm the patient’s immune response. Therefore, various drugs are being developed to overcome this problem and achieve a functional cure for CHB. To this end, toll-like receptor (TLR) agonists, retinoic acid-inducible gene (RIG) agonists, immune checkpoint inhibitors, therapeutic vaccines, genetically engineered T cells, and monoclonal antibodies are being explored.
TLR agonist
TLRs recognize viral and bacterial pathogen-associated molecular patterns (PAMPs) and induce downstream signaling pathways that culminate in the transcriptional activation of interferon-stimulating genes. GS-9688 (selgantolimod) is a TLR-8 agonist, and the interim results of the phase 2 clinical study in combination with NAs were recently presented. Weekly administration of 3 mg GS-9668 for 24 weeks decreased HBsAg titer by 0.5 log10 IU/mL or more in 7% of treatment-naïve patients (vs. 0% in the control group) and demonstrated functional cure in 5% of patients who were virologically stable [231,232].
Immune checkpoint inhibitor
HBV evades the HBV-specific immune response by inducing sustained expression of inhibitory receptors on T cells. Therefore, a functional cure for CHB can be achieved by overcoming the mechanisms underlying immune inhibition using an immune checkpoint inhibitor. A pilot study reported that single administration of nivolumab (anti-PD-1), a widely used anticancer drug, to patients with HBeAg-negative CHB significantly reduced serum HBsAg titer for 24 weeks. This showed the possibility of a functional cure using immune checkpoint inhibitors [233]. Envafolimab, developed as an anti-PD-L1, is undergoing a phase 2 clinical trial as a combination treatment with NAs, and the interim results were recently announced. Serum HBsAg titer decreased by 0.49 log10 IU/mL during a 12-week follow-up after a single dose of 2.5 mg Envafolimab [234].
Therapeutic vaccine
A therapeutic vaccine can enhance the host HBV-specific immune response through exposure to various antigens and proteins of HBV.
NASVAC is a nasal spray vaccine currently undergoing phase 3 clinical trials. During a follow-up period of 138 weeks, 6.3% of the previously NA-treated group and 21.7% of the treatment-naïve group showed a reduction in the serum HBsAg titer [235]. GS-4774, expressing HBs, HBc, and HBx antigens, with tenofovir DF did not show a significant decrease in serum HBsAg titer compared to the control group [236,237].
Combination treatment
Several drugs are being developed for the functional cure of CHB. In many phase 2 clinical trials, NA or peginterferon was used in combination with these drugs rather than being administered alone for effectiveness and safety. Accordingly, combinations of three or more classes of drugs are also being evaluated.
A previous study confirmed the reduction of serum HBsAg upon combined administration of entecavir, peginterferon, and HBV vaccine in patients with CHB who sustained virological response. The HBsAg loss rate of 16.2% at 100 weeks was significant compared to that in the control group [238].
In a recent pilot study, a combination of JNJ-3989, a siRNA agent; JNJ-6379, a capsid assembly modulator; and NAs was administered to the participants. All participants showed a decrease in serum HBsAg titer by 1.0 log10 IU/mL or more in 16 weeks, and the combination treatment was declared a breakthrough treatment [239]. Based on this observation, a phase 2 clinical trial was conducted. The recent interim result showed that the three-agent combination treatment was less effective with more adverse events than the two-agent combination treatment with JNJ-3989 and NAs [240]. Therefore, not all combinations are advantageous in terms of therapeutic and adverse effects, and further research is warranted to determine optimal drug combinations.
Summary
1. New drugs that can achieve a functional cure for CHB are under development. Further research is crucial to understand the usage, efficacy, and adverse effects of each drug alone or in combination.
CESSATION OF TREATMENT AND MONITORING AFTER ANTIVIRAL TREATMENT
Clinical biomarkers for treatment endpoint
The ultimate goal of hepatitis B treatment is to reduce mortality and increase survival by continuously suppressing the proliferation of HBV. This goal can only be achieved by complete eradication of HBV in the liver in the early stages of infection; however, cccDNA persists in the hepatocyte nucleus despite antiviral treatment, so it is difficult to expect complete elimination of HBV. Therefore, it is practically very difficult to determine the end of NA therapy. Alternative biomarkers that are readily measurable and reflect the achievement of treatment goals are needed when considering cessation of NA therapy. In clinical practice, ALT normalization, undetectable HBV DNA, HBeAg loss or seroconversion, and HBsAg loss or seroconversion have been used as treatment endpoints. Recently, studies that can predict sustained off-treatment response and determine cessation of NA therapy through serum HBsAg quantitative test, serum HBcrAg quantitative test, and serum HBV RNA have been introduced [118,124,241-243]. Identification of biomarkers to find the best candidates who can cease NA therapy without clinical relapse and can achieve sustained off-treatment response or HBsAg loss is an unmet clinical need. When stopping NA, the virological relapse of HBV is reported to be around 20–70%, although there are differences depending on the patient’s condition and follow-up period [244-249]. Meanwhile, there are studies that the possibility of a functional cure, which is HBsAg loss, can increase as the immune response increases with ALT flare after cessation of NA therapy. Especially, HBsAg loss is reported to increase progressively with a higher probability in Caucasian patients with HBeAg-negative CHB who cease NA therapy [244,245,250]. Therefore, cessation of treatment should be carefully decided in consideration of safety and expected off-treatment response. Closing monitoring is recommended in all patients who discontinue NA. In particular, in cirrhotic patients, if clinical relapse occurs, there is a risk of acute exacerbation, hepatic decompensation, and death, so special attention is required [144,151,251,252].
The standard treatment duration of peginterferon alfa is 48 weeks [253,254]. However, there have been reports that extended dosing could be more effective in HBeAg-negative CHB [255].
ALT normalization
Normalization of ALT in CHB treatment reflects a decrease in hepatic inflammatory response, mostly associated with undetectable HBV DNA, and reduces clinical deterioration [166]. Normalization of ALT during treatment reflects improvement in cirrhosis and could be considered reflective of treatment goals. However, 14–40% of patients with persistently normal ALT could have significant fibrosis (≥F2), and there is a variety of concurrent liver conditions affecting ALT level, such as non-alcoholic or alcoholic fatty liver [198,256]. As such, ALT normalization alone is insufficient when determining the endpoint of treatment.
Undetectable HBV DNA
HBV DNA level is the strongest indicator of disease progression and long-term outcomes in the natural course of CHB [68,70]. HBV DNA level is associated with histological activity in CHB patients, with low rate of progression to decompensation and high rate of survival in patients with low HBV DNA [257,258]. Antiviral therapy can reduce HBV DNA level, and histological improvement can be achieved in proportion to HBV DNA reduction [198,258-260]. In addition, since it reduces the progression and exacerbation of liver disease and prevents HCC [261,262], HBV DNA is a useful alternative indicator that reflects treatment endpoints. When HBV DNA is not detectable over a long term, and virological response is well maintained, the HBsAg loss rate increases even after cessation of therapy in patients with HBeAg-negative CHB. Therefore, cessation of therapy could be considered in these patients with long-term undetectable HBV DNA [244-246,250]. The lower is the HBV DNA level, the better is the clinical status; however, when the HBV DNA level is 60–2,000 IU/mL, the risk of cirrhosis and HCC is similar to that of patients with undetectable HBV DNA [68,70]. Evidence for HBV DNA level as a surrogate indicator in these patients is lacking. Also, in practice, most patients with undetectable HBV DNA relapsed after cessation of NA therapy [194,244-246]. Hence, undetectable HBV DNA cannot be the sole indicator determining treatment cessation.
HBeAg loss and/or seroconversion
HBeAg seroconversion in patients with HBeAg-positive CHB is accompanied by HBV DNA reduction, ALT normalization, and histological improvement. After HBeAg seroconversion, HBsAg loss increases to 1.15% per year [263,264]. Therefore, HBeAg loss/seroconversion in HBeAg-positive CHB could be an indicator reflecting achievement of the treatment goal. However, after cessation of NA therapy following HBeAg loss/seroconversion, the sustained virological response is reported to be 62.5%, 53.4%, and 51.5% at 1, 2, and 3 years, respectively [265]. Also, in some patients, HBeAg-negative CHB and HBeAg reversion or acute exacerbation accompanied by jaundice can occur in severe cases [266], so there is a limit to the indicator determining treatment cessation with only HBeAg loss/seroconversion. Sustained undetectable HBV DNA after HBeAg loss/seroconversion and the duration of consolidation therapy to maintain ALT normalization are important for maintaining response after cessation of NA therapy [248,267], but studies on the specific period are lacking. Nevertheless, it is recommended to maintain consolidation therapy for at least 12 months after HBeAg loss/seroconversion [144,151,252,265,268].
Quantitative HBsAg level, quantitative HBcrAg level, and HBV RNA
HBsAg is translated from pre-S1 and S2 messenger RNAs that are transcribed from the S gene. HBsAg exists as three protein subtypes (small, middle, and large) that are not differentiated by commercial assays. Additionally, there are two non-infectious subviral particles secreted, in spherical and filamentous forms, with 100-fold to 100,000-fold higher levels than mature virions [104]. The level of HBsAg has been found to be an indicator for off-therapy sustained response in many studies [269-274]. The degree of decline in HBsAg level during peginterferon can predict the efficacy of treatment-induced immune response. In patients whose HBsAg level does not sufficiently decrease in the early phase of peginterferon, additional treatment is unlikely to be successful, so it is used as the stopping rule, reducing unnecessary treatment [269]. Additionally, HBsAg level can be useful to determine if cessation of NA therapy is an option [270-274]. Chen et al. found the HBsAg level at the end-of-treatment (EOT) was the most important predictor for HBsAg loss and sustained response after stopping lamivudine treatment. At EOT, the HBsAg cutoff value of 300 IU/mL could predict 55.6% HBsAg loss in HBeAg-positive patients. In HBeAgnegative patients, the HBsAg cutoff values of 120 and 200 IU/mL could predict 79.2% HBsAg loss and 93.3% post-treatment sustained response, respectively [271]. In a Taiwanese study of patients with EOT HBsAg level <100 IU/mL, the cumulative rates of clinical relapse and sustained virological response were 9.3% and 45.5%, respectively, during a mean 2-year follow-up period after cessation of entecavir treatment [272]. In a large retrospective study of 691 HBeAg-negative patients who had discontinued NAs, during a median follow-up period of 156 (2–614) weeks, virological and clinical relapse rates were reported in 79.2% and 60.6%, respectively. In this study, EOT HBsAg level <100 IU/mL was an independent factor for HBsAg loss [273]. Another study showed EOT HBsAg level to be an independent predictor of virological and clinical relapse in HBeAg-negative patients who had discontinued tenofovir DF treatment, and HBsAg level of 80 IU/mL was the optimal value. The virological and clinical relapse rates at 78 weeks were 19.6% and 15.4%, respectively, in patients who achieved EOT HBsAg ≤80 IU/mL. Also, the cumulative rate of HBsAg loss at 104 weeks were 45.5% in these patients [274]. As such, low EOT HBsAg level (10–200 IU/mL) has been reported to be a good predictor of sustained virological response and HBsAg loss after cessation of NA therapy. Therefore, monitoring of quantitative HBsAg level during NA therapy could be helpful in practice [243,270,273].
HBcrAg contains three products encoded by the PC/core gene, HBcAg, p22cr, and HBeAg, all of which can be measured by serological testing. Serum HBcrAg level is closely associated with intrahepatic total HBV DNA and cccDNA, as well as serum HBV DNA [104,113,118,241]. HBcrAg level is useful for predicting off-treatment sustained response after cessation of NA therapy. Recently, HBcrAg level alone or a combination of HBsAg levels has been proposed [247,275]. SCALE-B score (HBsAg level [S], HBcrAg [C], age [A], ALT [L], and tenofovir [E] used for HBV [B]) including HBsAg and HBcrAg levels at NA discontinuation was suggested for prediction of post-NA relapse and HBsAg loss in Asian CHB patients [276-278]. The scoring criteria for including HBsAg and HBcrAg levels for cessation of NA therapy have been proposed and incorporated into the Japan Society of Hepatology Guidelines for the Management of HBV infection [117].
HBV RNA has been introduced as a novel biomarker for cessation of NA therapy [104,242]. There are also studies that predict off-treatment response with a combination of other indicators, such as HBsAg level or HBcrAg level [119,124,279]. However, the value of this indicator has not yet been evaluated as a standardized test, and it is necessary to explore the role of HBV RNA in predicting outcomes after cessation of NA therapy.
HBsAg loss
HBsAg loss is defined as a sustained loss of HBsAg and HBV DNA from serum, with or without anti-HBs seroconversion [37]. HBsAg loss is considered “functional cure” as the optimal treatment endpoint from a virological and clinical point of view. The prognosis following spontaneous HBsAg loss is excellent, except in patients with cirrhosis or those with concurrent other viral infection [23]. The incidence of HCC is significantly reduced when HBsAg loss occurs before ages 45–50 years [280]. Some patients with HBsAg loss during antiviral therapy showed HBsAg reversion or low but detectable HBV DNA, but most patients maintain HBsAg loss and undetectable HBV DNA, and their incidence of liver complications, including HCC, is significantly lower compared to that of patients without HBsAg loss [46,47,49,281]. Therefore, HBsAg loss is generally regarded as the optimal outcome of antiviral therapy in CHB, reflecting the treatment goal [37], at which point NAs can be discontinued [46,281,282]. Recently, it has been reported that HBsAg reversion and serum HBV DNA detection are rare if antiviral therapy is discontinued after HBsAg loss, defined as testing HBsAg negative on two separate occasions at least 6 months apart [281,282].
Monitoring after antiviral treatment
Although off-treatment response can persist after cessation of NA therapy, clinical relapse can occur in some patients, and there is a risk of acute hepatitis flare, liver decompensation, or fulminant hepatitis. Therefore, regular monitoring of liver function tests, HBeAg, anti-HBe, and HBV DNA is needed to evaluate the durability of the treatment response, relapse, and deterioration in liver function. In particular, if serum HBV DNA increases after cessation of NA therapy, a more intensive monitoring plan should be implemented to determine whether NA should be re-administered [243]. HBsAg level measurement can help monitor HBsAg reduction or loss in patients without HBsAg loss on cessation of NA therapy [104,269,270]. Even in patients in whom HBsAg loss has been achieved, there is the potential risk for reversion of HBsAg or development of HCC [46,49,131,280-282]. Therefore, serum HBsAg and/or anti-HBs should be monitored, and continuous HCC surveillance should be performed.
[Recommendations]
1. Cessation of NA therapy is recommended after serum HBsAg loss in CHB patients (A1).
2. In HBeAg-positive CHB patients, cessation of NA therapy could be considered at least 12 months after HBV DNA is undetectable and serum HBeAg loss or seroconversion has been achieved (B2).
3. Long-term treatment should be considered in patients with liver cirrhosis. Indefinite NA therapy is recommended in patients with decompensated liver cirrhosis (B1).
4. Peginterferon alfa is administered for 48 weeks (A1).
5. With reference to quantitative HBsAg level, cessation of NA therapy could be considered (B1).
6. Biomarker test such as HBcrAg and HBV RNA can be performed when considering cessation of NA therapy (B2).
7. Liver function testing and serum HBV DNA measurement at 1- to 6-month intervals and HBeAg/anti-HBe testing at 3- to 6-month intervals are recommended during the first year after cessation of antiviral treatment. Liver function testing and serum HBV DNA measurement at 3- to 6-month intervals and HBeAg/anti-HBe testing at 6- to 12-month intervals are recommended if treatment response is maintained beyond one year after antiviral therapy (B1).
8. If virological response is maintained after cessation of NA therapy, follow-up HBsAg/anti-HBs testing should be performed to confirm HBsAg loss, maintenance, or reversion (B1).
MANAGEMENT IN SPECIAL CONDITIONS
Patients with HCC
The aims of antiviral treatment in patients with HBV-related HCC are suppression of HBV replication to prevent the progression of hepatic dysfunction, thereby enabling active treatment of HCC, and reduction of HCC recurrence after curative treatment.
HBV treatment in patients undergoing curative treatment for HCC
A recent meta-analysis demonstrated that the pooled rates of HBV reactivation and biochemical reactivation were 20% and 9%, respectively, in patients who underwent surgical resection for HCC without NA treatment [283]. In contrast, patients receiving antiviral prophylaxis had a pooled HBV reactivation rate of 2–4%. In terms of local ablation therapies such as radiofrequency ablation or percutaneous ethanol injection, the HBV reactivation rate in patients who received no prophylactic NA was 5–9%, whereas HBV reactivation was rarely diagnosed in those who received prophylactic NA therapy [283-286]. In HBsAg-positive HCC patients with undetectable serum HBV DNA level at HCC diagnosis, some retrospective studies in Korea and China reported that 22–33% of patients experienced HBV reactivation after surgical resection [287-290]. The risk of HBV reactivation was lower for those receiving NA.
Several studies have reported that antiviral treatment was associated with lower risk of tumor recurrence after curative treatment for HBV-related HCC. A Taiwanese large-scale retrospective study showed that patients who underwent NA treatment with entecavir, lamivudine, telbivudine, etc. showed a significantly lower risk of tumor recurrence or overall death after surgical resection for HCC compared to those not treated with these drugs, although the prevalence of cirrhosis was significantly higher [291]. A Chinese randomized controlled trial (RCT) to evaluate the effect of NA treatment on postoperative prognosis of HBV-HCC showed that antiviral treatment significantly decreased HCC recurrence (hazard ratio [HR], 0.48; 95% confidence interval [CI], 0.32–0.70) and HCC-related death (HR, 0.26; 95% CI, 0.14–0.50) [292]. Even in RCTs including patients with low-level viremia (HBV DNA <2,000 IU/mL), antiviral treatment was associated with longer recurrence-free survival and overall survival [293]. NA therapy was also associated with a decreased risk of HCC recurrence among patients with HBV-related HCC post-RFA (HR, 0.69; 95% CI, 0.50–0.95) [294]. A meta-analysis showed that the antiviral treatment group had significantly lower risk of tumor recurrence (odds ratio [OR], 0.59; 95% CI, 0.35–0.97), liver-related mortality (OR, 0.13; 95% CI, 0.02–0.69), and overall mortality (OR, 0.27; 95% CI, 0.14–0.50) than the no antiviral treatment group [295].
Regarding the choice of oral antivirals, a Korean retrospective study demonstrated that antiviral treatment with high-potency NAs (i.e., entecavir and tenofovir) showed significantly longer recurrence-free survival than both antiviral treatment with low-potency NAs (i.e., lamivudine, clevudine, and telbivudine; HR, 0.47; 95% CI, 0.34–0.65) and no antiviral treatment (HR, 0.39; 95% CI, 0.30–0.51) [296]. However, it is not clear whether tenofovir and entecavir have different effects on HCC recurrence in patients receiving curative hepatectomy for HBV-related HCC. Recent Korean retrospective study reported that tenofovir DF therapy was associated with a significantly lower risk of HCC recurrence after curative resection and better overall patient survival compared with entecavir [297]. In contrast, no difference in the risk of death or liver transplantation was observed in a Taiwanese retrospective cohort with hepatectomy for early-stage HCC, despite tenofovir DF therapy being associated with a lower risk of HCC recurrence than entecavir [298]. The prognoses in terms of recurrence and death after curative treatment of HBV-related HCC were not statistically different between the entecavir and tenofovir DF groups in a multi-center retrospective study in Korea [299].
HBV treatment in patients undergoing locoregional therapy for HCC
After transarterial chemoembolization (TACE), approximately 4–40% of patients with HBV-related HCC developed HBV reactivation [283,300-304]. Post-TACE risks of HBV reactivation, flare-up hepatitis, and liver failure due to HBV reactivation were 2.8%, 2.8%, and 0%, respectively, in the prophylactic lamivudine treatment group and 40.5%, 29.7%, and 8.1% in the control group. There was a significant difference between the two groups [302]. After hepatic artery infusion chemotherapy (HAIC), HBV reactivation was reported in 24–67% of patients, which was relatively higher than that after TACE. This finding can potentially be explained by a larger total amount of cytotoxic chemotherapeutic agents due to shorter treatment intervals than TACE [305-307]. Furthermore, prophylactic antiviral treatment is significantly associated with improved long-term survival among patients undergoing transarterial chemotherapy, including TACE and HAIC (10-year overall survival, 26.5% vs. 12.8%) [203]. The prospective Chinese study involved 98 patients with HBsAg-positive/HBV DNA-negative HCC reported that HBV reactivation occurred in 23.4% of patients in the nonantiviral group (11/47, 23.4%), but only 5.9% of patients in the antiviral group (P<0.05) [308].
In patients who underwent external beam radiation therapy (EBRT) for HCC, reactivation and ALT elevation were reported in 0% and 2.3%, respectively, of the lamivudine-prophylaxis group and 21.8% and 12.5% of the control group. The control group had a significantly higher risk of HBV reactivation [283,309]. A recent Korean retrospective study also showed that the absence of antiviral treatment was an independent risk factor for HBV reactivation in EBRT for HCC (OR, 8.34; 95% CI, 2.53–27.47) [310]. Therefore, preventive antiviral therapy is recommended for patients with HCC who are scheduled to receive EBRT. The combination treatment with TACE and EBRT had twice the risk of HBV reactivation compared to TACE treatment alone [311]. In addition, either TACE or combination treatment of TACE with EBRT can reactivate HBV replication in HBsAg-negative/anti-HBc-positive individuals [312,313]. Patients with prior chronic HBV infection who undergo intensive TACE should be closely monitored, with an alternative approach of antiviral prophylaxis against HBV reactivation.
HBV treatment in patients undergoing systemic therapy/immune checkpoint inhibitors for HCC
HBV reactivation was diagnosed in 6.2% of patients treated with sorafenib based on the data of a recently published meta-analysis [283]. A few retrospective studies showed that underlying HBV DNA >2,000 IU/mL was associated with poor prognosis in sorafenib-treated patients, whereas antiviral prophylaxis was significantly related to the improvement of overall survival [314,315].
Immune checkpoint inhibitors can enhance host immunity and consequently have a lower risk of HBV reactivation. However, immune checkpoints can result in severe acute aggravation of hepatitis since it can upregulate antiviral immunity against HBV. Therefore, suppression of HBV replication with antiviral treatment is necessary before use of immune checkpoint inhibitors [316]. The overall pooled HBV reactivation rate was 7.8% in HCC patients receiving immune checkpoint inhibitors, and most of them did not receive antivirals [283]. A Korean retrospective study including 398 HBV-HCC patients receiving both immune checkpoint inhibitors and antiviral prophylaxis demonstrated that two (0.5%) showed HBV reactivation, both of whom showed poor adherence to antivirals [317]. In addition, retrospective studies reported that antiviral prophylaxis is less likely to reactivate HBV regardless of baseline HBV DNA level [318,319]. Thus, antiviral prophylaxis is recommended in HCC patients receiving immune checkpoint inhibitors.
In atezolizumab and bevacizumab combination treatment or lenvatinib, which have recently been approved as the first-line treatment for liver cancer, further studies are needed due to the lack of data on HBV reactivation.
[Recommendations]
1. In patients with HBV-related HCC, antiviral therapy should be initiated if serum HBV DNA is detected (A1).
2. In patients with HBsAg-positive HCC who undergo anticancer treatment, prophylactic antiviral therapy with NAs should be considered regardless of detectable serum HBV DNA (B1).
3. HBV reactivation should be monitored in patients with past HBV infection undergoing anti-HCC treatment, especially TACE or EBRT (B2).
Patients with renal dysfunction or metabolic bone disease
Long-term administration of adefovir or tenofovir DF in the patients with CHB can result in decreased renal function and bone mineral density. Side effects such as acute or chronic renal failure, hypophosphatemia, and Fanconi syndrome have been reported [320-323]. If patients already have risk factors for renal dysfunction and/or metabolic bone disease, or if worsening kidney function or bone disease is detected during treatment, a change in treatment regimen needs to be considered.
Patients with renal dysfunction or metabolic disease prior to starting treatment
Patients with chronic kidney disease have relatively higher rate of exposure to HBV infection [324]. In Korea, a 4–7% HBsAg-positive rate has been reported among hemodialysis patients [325-329]. When starting NA treatment in patients with chronic kidney disease, the dose must be adjusted according to the creatinine clearance (Table 8). Tenofovir alafenamide fumarate (tenofovir AF) and tenofovir DF are not recommended in patients with creatinine clearance below 15 and 10 mL/min, respectively, without renal replacement. This is also true for besifovir in cases of creatinine clearance below 15 mL/min.
Because NA treatment itself can affect renal function or bone density, it is necessary to select an appropriate drug if there is any risk factor such as long-term steroid use. In addition, patients treated with tenofovir DF should be monitored for bone mineral density during treatment. In a large phase 3 trial comparing tenofovir AF and tenofovir DF over a 96-week treatment period, among those with any risk factor for renal dysfunction (age over 50 years, hypertension, cardiovascular disease, diabetes, or hyperlipidemia), patients treated with tenofovir DF, compared to patients treated with tenofovir AF, had worsening renal function (compared to baseline, median changes in estimated glomerular filtration rate [eGFR] were -5.0 mL/min and -0.3 mL/min, respectively) [330-332]. Additionally, among those with a risk factor for decreasing bone density (female, age over 50 years, Asian, and baseline eGFR <90 mL/min) patients treated with tenofovir DF showed a greater decrease in bone mineral density compared to patients treated with tenofovir AF (-3.29% and 1.23%, respectively) [330-332]. Therefore, it is recommended to avoid the use of tenofovir DF among patients with risk factors for renal dysfunction such as baseline eGFR <60 mL/min, proteinuria, albuminuria (urine albumin: creatinine ratio >30 mg/g), hypophosphatemia (<2.5 mg/dL), uncontrolled diabetes, or hypertension. If patients have a diagnosis of osteopenia or osteoporosis, need to be on chronic steroid treatment, or take other medications that can lower the bone density, other NAs that more weakly affect bone density should be considered over tenofovir DF (Fig. 5) [151].

Indications for selecting entecavir, tenofovir alafenamide fumarate, or besifovir over tenofovir disoproxil fumarate. AF, alafenamide fumarate. *Dose was adjusted if creatinine clearance <50 mL/min, refer to Table 8. †Not indicated if creatinine clearance <15 mL/min without dialysis. ‡Not indicated if creatinine clearance <15 mL/min.
In addition to tenofovir AF, entecavir and besifovir have less of an effect on renal function and bone metabolism. Besifovir, recently approved for use, had been evaluated in a clinical trial for safety in reduction of renal function and bone density. In a multicenter phase 3 trial from Korea, the median decrease from baseline in eGFR was -0.5 mL/min in patients with besifovir and -7.8 mL/min in patients with tenofovir DF. In terms of bone mineral density, patients treated with besifovir showed median decreases from baseline in bone mineral density in spine and hip of 0.33% and 0.44%, respectively, whereas patients treated with tenofovir DF had median decreases of 0.85% and 1.29% [333]. Therefore, besifovir is thought to have a better effect on renal function or bone density compared to tenofovir DF. However, besifovir is not indicated in cases of severely decreased renal dysfunction (eGFR <15 mL/min) due to lack of clinical data.
Patients who developed renal dysfunction or decrease in bone density on treatment with NAs
If patients develop renal dysfunction or decrease in bone density while on NAs, the causative factors must be identified and corrected. Then, the dose must be modified accordingly (Table 8) or a review should be performed for drug change (Fig. 5).
A phase 3 clinical trial compared the safety of tenofovir AF to that of tenofovir AF during 240 weeks. In this trial, patients were given either treatment for 96 weeks each, after which some patients treated with tenofovir DF switched to tenofovir AF, while the remaining switched to tenofovir AF at 144 weeks. Patients who switched from tenofovir DF to tenofovir AF at 96 weeks (tenofovir AF for 48 weeks afterward) showed improved renal function at 144 weeks compared to patients who continued tenofovir DF at 144 weeks (compared to baseline, mean change in eGFR: 4.2 mL/min and -0.9 mL/min, respectively). In terms of bone mineral density in spine and hip, patients who were treated with tenofovir DF showed greater decreases than patients treated with tenofovir AF until week 96. However, after switching to tenofovir AF at week 96 or week 144, bone mineral density recovered to baseline at week 240 in these patients, resulting no significant difference in patients initially treated and then switched and those who maintained tenofovir AF for 240 weeks [334].
In another phase 3 non-inferiority switching trial, patients who switched to tenofovir AF from tenofovir DF had improvements in renal function (compared to baseline, median change 0.94 mL/min vs. -2.74 mL/min, respectively) and bone mineral density (compared to baseline, median change 0.66% vs. -0.51% [hip]; 1.74% vs. -0.11% [spine]) compared to patients who continued tenofovir AF at week 48 [335]. Additionally, in a randomized non-inferiority trial of patients with multidrug-resistant HBV, patients who switched to tenofovir AF from tenofovir DF, compared to patients continuing tenofovir AF, showed a greater increase in median eGFR (compared to baseline, 7.3% vs. 1.9%, respectively) and an improvement in bone mineral density (compared to baseline, 1.8% vs. 0.2%, respectively) [336]. Therefore, it is thought that reduction in renal function and/or bone density while on tenofovir DF can be improved by switching to tenofovir AF.
In a phase 3 trial comparing besifovir with tenofovir D, patients receiving tenofovir DF for 48 weeks showed a decrease in eGFR by -7.8 mL/min. However, renal function as measured by eGFR was recovered to baseline after switching to besifovir and continuing to 144 weeks. In terms of bone mineral density in spine and hip, patients receiving tenofovir DF showed decrease of 1.12% and 0.62%, respectively. After switching to besifovir, bone density was improved and recovered to baseline, showing no significant difference from patients who were initially treated with besifovir. These improvement in renal function and bone density were maintained after 144 weeks of besifovir treatment [337,338]. Therefore, during the NA treatment of CHB, if patients develop renal dysfunction or metabolic bone disease, and/or carry risk factors, appropriate drug change can be an option for overcoming the side effects (Fig. 5).
[Recommendations]
1. Entecavir, tenofovir AF, and besifovir are preferred over tenofovir DF in treatment-naïve CHB patients with or at risk of renal dysfunction or metabolic bone disease (A1).
2. Treatment can be switched to tenofovir AF, besifovir, or entecavir depending on treatment history in patients on tenofovir DF with or at risk of renal dysfunction or metabolic bone disease (A1).
3. NA dose should be adequately adjusted for creatinine clearance (A1).
Patients on immunosuppression or chemotherapy
The progression of CHB is determined by the interaction between the virus and host immune response. Therefore, if the immune response is suppressed by immunosuppressive therapy or anticancer chemotherapy, the risk of reactivation increases [339].
Reactivation of CHB
Reactivation of hepatitis B indicates recurrence of active necrotizing inflammatory disease in patients in the inactive phase of CHB or those who recovered from previous active infection. Such reactivation can be largely divided into two categories, “exacerbation of chronic HBV infection” for those with positive HBsAg and “relapse of past HBV infection” for those with negative HBsAg and positive anti-HBc [340]. In the latter category, patients who remained in an “occult HBV infection” status can show viral replication triggered by immunosuppression, leading to reverse seroconversion or seroreversion, with redetection of HBsAg [341-343]. Exacerbation of chronic HBV infection is defined in those with seropositive HBsAg as an increase of serum HBV DNA by more than 100 times the baseline level. Relapse of past HBV infection is defined as seroconversion of HBsAg-negative to positive or detection of serum HBV DNA from undetectable level to greater than 100 IU/mL. Hepatitis flare due to HBV reactivation is defined as a ≥3-fold increase in serum ALT level, to a total exceeding 100 IU/L [344,345].
Various rates of reactivation have been reported but are about 20–50% in HBsAg-positive patients receiving anti-cancer chemotherapy. For an accurate diagnosis, liver damage related to chemotherapy, tumor metastasis, or hepatitis secondary to other viruses should be excluded. In many cases, patients are asymptomatic but occasionally present with jaundice or are in various stages such as decompensated liver disease or death [343,346-348]. Typical reactivation is noted by detection of serum HBV DNA during immunosuppression or chemotherapy or elevation of serum ALT after stopping the immunosuppressive therapy. If the reactivation occurs during chemotherapy, it can lead to reduction or discontinuation of chemotherapy, adversely affecting treatment success [349-351]. There are risk factors of hepatitis B reactivation related to the virus, the host, and treatment. Virus factors include serum HBV DNA, HBeAg seropositivity, hepatocyte cccDNA, and PC/BCP mutation prior to treatment; host factors include type of malignant tumor, male gender, young age, and high serum ALT level; and treatment factors include the type and intensity of immunosuppressant or chemotherapy regimen, hematopoietic stem-cell transplantaton (HSCT), and/or solid organ transplantation [352]. The type and intensity of chemotherapy regimen related to the risk of hepatitis B reactivation can be classified into three categories: high risk group (reactivation risk of 10% or more), moderate risk group (reactivation risk between 1–10%), and low risk group (reactivation risk less than 1%) (Table 9) [316,353,354].
Reactivation of hepatitis B during chemotherapy for lymphoma and other hematologic malignancies
During chemotherapy for lymphoma, hepatitis B reactivation is reported to be frequent, with the rate up to 24–67%. This not only implies that the chemotherapy used for lymphoma is strong enough to cause bone marrow suppression, but also that patients with lymphoma have higher rates of seropositive HBsAg than those without lymphoma [347,355-357]. Rituximab, commonly used in combination with steroids for the treatment of lymphoma, is known to increase the risk of reactivation [358,359]. Rituximab therapy increased the risk of hepatitis B reactivation in patients with non-Hodgkin’s lymphoma who had seropositive HBsAg or seronegative HBsAg/seropositive anti-HBc combination (relative risk [RR], 2.14; 95% CI, 1.42–3.22; P<0.001). Even in patients with seronegative HBsAg/seropositive anti-HBc combination, the use of rituximab therapy was associated with higher risk of reactivation (RR, 5.52) [360]. In a retrospective observational study of lymphoma patients treated with rituximab, hepatitis B reactivation rate was 27.8% in patients with seropositive HBsAg, and the risk could be lowered by the prophylactic antiviral treatment (22.9% [32/140] vs. 59.1% [13/22]; P<0.001). However, hepatitis B reactivation developed in more than 20% of patients who received prophylactic antiviral treatment. In a retrospective study, hepatitis B reactivation rate was as low as 2.4% in patients with seronegative HBsAg/seropositive anti-HBc combination [361]. According to a recent meta-analysis, this rate was 17% in prospective studies and 7% in retrospective studies [362]. In addition, in a prospective observational study of lymphoma patients with seronegative HBsAg/seropositive anti-HBc combination who were treated with rituximabCHOP (R-CHOP) chemotherapy, hepatitis B reactivation and subsequent worsening of hepatitis were common (10.4 vs. 6.4 per 100 person-year). In that study, there were cases where hepatitis worsened during periodic monitoring of HBsAg and serum HBV DNA, and immediate antiviral treatment was safe for reactivation of hepatitis B. In particular, seroreversion of HBsAg was the most important indicator of hepatitis B-related liver damage (100% vs. 28%) [363]. There was a significant difference in the reactivation of hepatitis B in patients with and without prophylactic antiviral therapy (13.3% vs. 60%) during treatment with rituximab [364]. Furthermore, prior to receiving chemotherapy (R-CHOP), screening for hepatitis B in all patients, rather than limiting to high-risk groups, resulted in a 10-fold reduction in hepatitis B reactivation rate and economic and survival benefits [365]. Lamivudine was the most commonly used antiviral agent, but the hepatitis B reactivation rate was significantly lower in the entecavir group than in the lamivudine group (6.3% vs. 39.3%; P<0.05). In a recent, prospective, randomized control study, hepatitis B reactivation was reported to be 0% in the group treated with prophylactic tenofovir DF during rituximab treatment but 10.7% (P=0.09) in the group not receiving prophylactic antiviral therapy [366].
With other hematologic malignancies, if patients are receiving high-intensity chemotherapy prior to HSCT, the risk of reactivation is high [366,367]. In particular, in patients with seropositive HBsAg or seronegative HBsAg/seropositive anti-HBc awaiting high intensity chemotherapy prior to HSCT, antiviral therapy with a high barrier to resistance is recommended [368]. During immunosuppressive therapy or chemotherapy for hematologic disorders, for patients with evidence of hepatitis B infection, prophylactic treatment with lamivudine or entecavir can significantly lower the reactivation rate [369,370].
Meanwhile, in a recent Korean study evaluating 93 multiple myeloma patients with seronegative HBsAg who were treated with daratumumab (anti-CD38), hepatitis B reactivation occurred in 6 (6.5%, 6/93). Subgroup analysis for patients with seronegative HBsAg/seropositive anti-HBc combination showed a higher risk of 12.5% (3/24). Furthermore, special attention is needed because one patient eventually died due to liver failure even though all six hepatitis B-reactivated patients were treated with prompt antiviral therapy [371].
Reactivation of hepatitis B during chemotherapy for solid tumors
Reactivation of hepatitis B in patients with solid tumors occurs in 14–21%, while that of breast cancer is higher at about 41– 70%, which is thought to be related to the high doses of treatment agents and the use of anthracycline-based chemotherapy and steroids [351,372,373]. Steroids not only suppress the immune system, but directly stimulate the replication of HBV, increasing the risk of reactivation. Use of prophylactic antiviral agents in most solid tumors, not only breast cancer, but also lung cancer in which immunotherapy is being used as a first-line treatment, has significantly reduced the rates of hepatitis B reactivation and discontinuation of chemotherapy treatment [374,375].
Reactivation of hepatitis B during immune-related treatments for inflammatory bowel disease (IBD) or rheumatoid arthritis (RA)
The reactivation of hepatitis B also might be associated with the use of tumor necrosis factor-alpha (TNFα) inhibitors (infliximab, etanercept, adalimumab, etc.) for the treatment of IBD or RA [375-380]. For TNF α inhibitors used for the treatment of IBD, hepatitis B reactivation occurred in 22.5–39% of patients with seropositive HBsAg and 5% of seronegative HBsAg/seropositive anti-HBc combination [381]. In cases treated with TNFα inhibitors and disease-modifying antirheumatic drugs (DMARDs) for RA treatment, the rate of reactivation of hepatitis B was around 12.3% in patients with seropositive HBsAg [382]. In another study, reactivation was reported in 39% of HBsAg-positive patients and 5% of antiHBc-positive patients; among those given antiviral prophylaxis, the reactivation rate was significantly lower (23% vs. 62%; P=0.003) [383]. In a meta-analysis of patients treated for RA, hepatitis B reactivation occurred in 14.6% of HBsAg-positive patients and was lower at 9.0% in those treated with prophylactic antiviral therapy [384]. However, the reactivation rate tended to decrease in the TNFα inhibitor treatment group (4.4% vs. 15.6%; P=0.05), but no significant difference was observed from the DMARD treatment group (27.1% vs. 22.4%) [384]. This finding might be related with the high heterogeneity of studies included in this metaanalysis, and further studies are needed. In the same study, hepatitis B reactivation occurred in 1.6% of cases where only anti-HBc was positive [384].
Acute exacerbation of hepatitis B during immunotherapy treatment
Recently, immune checkpoint inhibitors such as anti-PD-1 (nivolumab), anti-PD-L1 (atezolizumab), and anti-CTLA4 (ipilimumab) have been used in various carcinomas including liver cancer. According to a recent retrospective study analyzing patients with multiple solid cancers and some lymphomas, the incidence rates of hepatitis B reactivation of the total patients, HBsAg-positive patients, and HBsAg-negative patients were 0.14% (5/3,465), 1.0% (5/511), and 0.0% (0/2,954), respectively. The hepatitis B reactivation rates were 0.4% (2/464) and 6.4% (3/47) in patients with and without antiviral prophylaxis, respectively [317]. Hepatitis B reactivation is rare in immune checkpoint inhibitor chemotherapy, but prophylactic antiviral therapy can be considered in HBsAgpositive patients. Pembrolizumab among various immune checkpoint inhibitors showed a significant correlation with hepatitis B reactivation [385]. In a retrospective study evaluating 89 patients with hematologic malignancies treated with chimeric antigen receptor (CAR)-T cells, all 19 HBsAg-positive patients received prophylactic antiviral therapy with entecavir, but hepatitis B reactivation occurred in one (5.3%). However, hepatitis B reactivation was not observed in patients with seronegative HBsAg/seropositive anti-HBc combination despite only two of 37 patients receiving prophylactic entecavir therapy. Furthermore, no hepatitis B reactivation occurred during prophylactic antiviral therapy in the 33 patients with seronegative HBsAg/seronegative anti-HBc combination. Therefore, prophylactic antiviral therapy might be beneficial to HBsAg-positive patients treated with CAR-T cells, who are thought to have higher than intermediate risk of hepatitis B reactivation [386]. There are concerns about the possibility of acute exacerbation of hepatitis B in relation to these treatments, but there is insufficient data, and further consideration of prophylactic antiviral therapy is needed.
Start and end points of prophylactic antiviral therapy
When the reactivation of hepatitis B occurs, there is a risk of liver failure or even death. Therefore, prevention is most important. Prior to starting an immunosuppressive therapy or chemotherapy, screening for HBsAg and anti-HBc is necessary. If there is no evidence of HBV infection in the past (with negative HBsAg and anti-HBc), HBV vaccination can be considered. In HBsAg-positive cases, regardless of serum HBV DNA level, antiviral prophylaxis is recommended. Instead of waiting for the serum HBV DNA level to rise, administering an antiviral agent at the start of the immunosuppressive therapy or chemotherapy or 7 days prior to the treatment start date is reported to be more effective [377,387-389]. The end point of the prophylactic antiviral treatment should theoretically be continued until the immune system is adequately recovered, but there is lack of sufficient evidence to suggest a specific end point. It has been reported that the risk of HBV reactivation is high when prophylactic lamivudine is discontinued about 3 months after the end of chemotherapy. The risk is especially higher when the serum HBV DNA prior to the treatment is elevated (≥2,000 IU/mL) [390]. Therefore, when HBV is actively replicating before prophylactic antiviral therapy, following the present CHB treatment guidelines for the discontinuation of antiviral agents might prevent virus reactivation after treatment. However, regardless of the serum HBV DNA level prior to the treatment, reactivation is reported more than 6 months after the completion of chemotherapy, so caution is required. Therefore, antiviral prophylaxis should be maintained for at least 6 months after the chemotherapy is completed, and extension should be considered according to the chemotherapy risk. Especially, for patients receiving chemotherapy involving rituximab, it is recommended to extend the antiviral prophylaxis to at least 12 months after the completion of chemotherapy [391-393]. Even after the end of prophylactic antiviral therapy, it is necessary to closely monitor for relapse for more than at least 12 months.
Meanwhile, as described above, hepatitis B reactivation requires attention because it can occur not only when HBsAg is positive, but also when HBsAg is negative and anti-HBc is positive. In particular, under immunosuppressive conditions, patients who were only positive for anti-HBc had a higher risk of hepatitis B reactivation than patients who were positive for both anti-HBc and anti-HBs [316,394]. Therefore, when HBsAg-negative and anti-HBc-positive patients are treated with a rituximab-containing regimen or HSCT for leukemia, it is necessary to consider prophylactic antiviral therapy because the risk of hepatitis B reactivation is greater than 10%. Meanwhile, in a Korean study analyzing the risk of hepatitis B reactivation in patients who received chemotherapy including rituximab for lymphoma, the risk of hepatitis B reactivation was low in patients with resolved HBV (HBsAg-negative and HBcAb-positive) when anti-HBs titer was high (>100 IU/mL). For these patients, meticulous follow-up for ALT and HBV DNA and prompt commencement of antiviral therapy upon hepatitis B reactivation are a possible strategy [395]. For patients receiving intermediate- or low-risk groups of chemotherapy, HBsAg and HBV DNA should be monitored periodically (every 1–3 months) during and after the chemotherapy, and antiviral treatment should be commenced upon hepatitis B reactivation [316].
Treatment medications
Lamivudine is the most widely studied drug for prophylactic antiviral therapy. It is well known to significantly reduce reactivation, liver failure, and death according to randomized controlled trials of lymphoma patients in Hong Kong and Taiwan [356,367,387,396]. However, lamivudine has been reported to be resistant even during prophylaxis. If the treatment duration is expected to be long, it is necessary to select a therapeutic agent with a high barrier to resistance considering the resistance rate [356]. In a retrospective study of lymphoma patients, the incidence of hepatitis and chemotherapy disruption due to HBV reactivation was significantly lower in the entecavir group than in the lamivudine group [397]. In a randomized controlled study comparing 121 patients treated with RCHOP chemotherapy for lymphoma as entecavir-treated (n=61) and lamivudine-treated groups (n=60), the entecavir group performed better in terms of the risk of hepatitis B reactivation (4% vs. 18%; P=0.001) and the rate of discontinuation of chemotherapy due to exacerbation of hepatitis B (1% vs. 11%; P=0.002) [398]. In a meta-analysis, entecavir prophylaxis was shown to prevent reactivation of hepatitis B more effectively than lamivudine prophylaxis [399]. In a retrospective comparative analysis of 419 CHB patients who received chemotherapy for solid cancers and lymphoma in Korea, HBeAg positivity, serum HBV DNA, and cancer types were identified as risk factors related to hepatitis B reactivation, and a risk stratification tool was developed based these factors. This study also confirmed that entecavir had a better preventive effect than lamivudine or telbivudine in the high-risk group [400]. However, since most prophylactic therapy studies were conducted on lymphoma patients, prospective studies evaluating various malignancies including solid cancers on appropriate antiviral agents and treatment duration for each cancer type and anticancer drug are needed. Considering the failure rate and tolerance rate, entecavir and tenofovir are expected to be relatively safe options. In a retrospective study evaluating patients receiving chemotherapy or immunosuppressant treatment for solid cancers, lymphoma, or rheumatic diseases, the tenofovir AF group (n=11) had similar effects compared to the entecavir group (n=66) in terms of HBV DNA reduction effect (-2.83±1.45 log IU/mL vs. -3.05±2.47 log IU/mL; P=0.86), HBV non-detection rate (78.8 vs. 90.9%; P=0.68), and renal function decline rate (-0.62±11.2 mL/min/1.73 m2 vs. -3.67±13.2 mL/min/1.73 m2; P=0.29), respectively [401]. Therefore, tenofovir AF can also be considered as a safe drug with good preventive effects. Such a conclusion regarding besifovir requires additional data. However, considering the characteristics of the drugs, entecavir, tenofovir AF, and besifovir are expected to be relatively safe choices as a prophylactic antiviral treatment for patients at risk or with renal and/or bone diseases.
[Recommendations]
1. If HBV infection is not confirmed, screening for HBsAg and anti-HBc before immunosuppression or chemotherapy is recommended. If either is positive, HBV DNA testing should be performed (A1).
2. If either HBsAg is positive or HBV DNA is detected, prophylactic antiviral therapy should be initiated before or at the start of immunosuppression or chemotherapy (A1). Antiviral agents should be selected based on comprehensive consideration of serum HBV DNA level, the intensity and duration of immunosuppression or chemotherapy, and presence or risk of renal and/or bone diseases. Entecavir or tenofovir (DF or AF) is preferred (B1), and besifovir could be an alternative option (C1).
3. In HBsAg-negative, HBV DNA-undetectable, and anti-HBc-positive patients, serum HBsAg and HBV DNA should be monitored during high-risk immunosuppression/chemotherapy and antiviral therapy started when HBV reactivation occurs (A1). In particular, when a regimen includes rituximab and/ or other B-cell depleting agents, antiviral therapy can be initiated at the start of immunosuppression or chemotherapy (B1).
4. Prophylactic antiviral therapy should be maintained for at least 6 months after the termination of immunosuppression or chemotherapy and for at least 12 months after the termination of therapy if rituximab and/or other B-cell depleting agents were included (B1).
5. Periodic monitoring of serum HBV DNA is recommended during and after prophylactic antiviral therapy (A1).
Hematopoietic stem cell transplantation
Patients with CHB who require HSCT for hematologic malignancies are immunosuppressed for a prolonged period due to the high-dose chemotherapy and hematological diseases itself. This increases the risk of hepatitis B reactivation and leads to a poor prognosis [402,403]. Therefore, all HSCT recipients should be tested for HBsAg, anti-HBc, and anti-HBs before transplantation. Quantitative serum HBV DNA test should be performed for patients who are HBsAg-positive/anti-HBc-positive or HBsAg-negative/anti-HBcpositive, regardless of whether they have anti-HBs or not. Hepatitis B vaccination should be administered and anti-HBs titers should be monitored for all hematologic malignancy patients who are HBsAg-negative/anti-HBs-negative. In small retrospective studies of HBsAg-positive recipients of allogeneic or autologous stem cell transplantation, prophylactic lamivudine treatment for 6–12 months significantly reduced the frequency of hepatitis B reactivation [367,404]. In another study, HBsAg-positive recipients of allogeneic stem cell transplants underwent prophylactic antiviral treatment for up to 6 months after termination of immunosuppressive therapy and were followed for 24 months after transplantation. The cumulative reactivation rate of hepatitis B was significantly higher in patients receiving lamivudine (24%) compared to patients receiving entecavir (2%). Recent meta-analyses have also demonstrated the efficacy of entecavir in preventing hepatitis B reactivation [405,406].
Hepatitis B reactivation is not infrequent in HSCT recipients with seronegative HBsAg/seropositive anti-HBc. In a prospective cohort study in which 62 HBsAg-negative and anti-HBc-positive allogeneic stem cell transplant recipients were followed for 48 weeks, the 2-year cumulative reactivation rate (detectable HBV DNA >10 IU/mL) was 40.8% [407]. In a retrospective study that followed HSCT recipients with past HBV infection in Korea for a median of 78 months, the hepatitis B reactivation rate was 2.6% (3/114). In another retrospective study with median follow-up of 21 months, hepatitis B reactivation occurred in four of 96 patients who received prophylactic antiviral therapy for 7 months and in 8 of 219 patients who did not receive prophylactic antiviral therapy [408,409]. According to observational studies, in HBsAg-negative and anti-HBc-positive allogeneic HSCT recipients, the 5-year cumulative incidence of hepatitis B reactivation is frequent (10.5–43.0%), so it is reasonable to commence prophylactic antiviral agents [410]. Various global guidelines recommend maintaining prophylactic antiviral treatment until 6–18 months after completion of HSCT; however, there is no unified consensus [144,151,368,411-413]. According to a recent study, hepatitis B reactivation was continuously reported up to 5–7 years after HSCT [410,414]. Therefore, it might be safe to maintain prophylactic antiviral therapy for a long time after the completion of HSCT, and further studies on the timing of discontinuation of prophylactic antiviral therapy are needed.
All HBsAg-positive solid organ transplantation and HSCT recipients should receive prophylactic antiviral therapy along with transplantation, and entecavir or tenofovir DF is preferred because long-term treatment is required. Although additional data are needed, given the characteristics of the drugs, entecavir, tenofovir AF, or besifovir could be recommended as a prophylactic antiviral treatment for patients at risk or with renal and/or bone diseases.
[Recommendations]
1. All HSCT recipients should be tested for HBsAg, anti-HBc, and anti-HBs before transplantation (A1).
2. Quantitative serum HBV DNA test should be performed for patients who are HBsAg-positive/anti-HBc-positive or HBsAg-negative/anti-HBc-positive, regardless of whether they have anti-HBs or not (B1).
3. Hepatitis B vaccination should be administered and anti-HBs titers should be monitored for all hematologic malignancy patients who are HBsAg-negative/anti-HBs-negative (C1).
4. All HBsAg-positive or HBV DNA-positive HSCT recipients should receive prophylactic antiviral therapy at the time of transplantation (A1). Antiviral agents should be selected based on comprehensive consideration of serum HBV DNA level, the intensity and duration of immunosuppression or chemotherapy, and presence or risk of renal and/or bone diseases. Entecavir or tenofovir (DF or AF) is preferred for long-term treatment (B1), and besifovir could be an alternative option (C1).
5. HBsAg-negative, HBV DNA undetectable, but anti-HBc-positive HSCT recipients are recommended to start prophylactic antiviral therapy at the time of transplantation (B1).
6. Prophylactic antiviral therapy should be maintained for at least 12 months after HSCT (B1).
Liver transplant patient
Prevention of HBV recurrence after liver transplantation is very important in patients receiving a liver transplantation. The current standard treatment is a combination of NA and hepatitis B immunoglobulin (HBIG), which lowers the posttransplant HBV reinfection rate to less than 5% [151,415]. Entecavir, tenofovir DF, and tenofovir AF are preferred antivirals because of their high potency and low rate of drug resistance [144,416,417]. As switching from other antiviral to tenofovir AF for HBV prophylaxis led to excellent virological response and a trend toward improvement in renal function in several recent studies, tenofovir AF is a reasonable option in posttransplant patients who have or are at risk for renal or bone disease [416,417]. With the advent of NA with high potency, efforts to reduce the duration of HBIG, which is expensive and inconvenient due to the parenteral route of administration, have been made in many transplantation centers [418-425]. In these studies, HBV recurrence rate was low at 0–13% when lifelong highly potent NAs were combined with a short period of HBIG (7 days to 1 year) after transplantation. In addition, HBIG-free prophylaxis using NA alone showed 8-year HBsAg negativity of 92% and 88%, respectively, in two studies, showing that NA monotherapy can effectively prevent posttransplant HBV recurrence [426,427]. NA monotherapy without HBIG has shown to be safe and effective in preventing HBV recurrence and transplant failure and warrants favorable long-term survival rate in liver transplant patients. Therefore, adjusting the period of HBIG use by evaluating the risk of HBV recurrence at the time of transplantation for each patient is recommended. Patients with a low risk of recurrence (HBV DNA negative at liver transplantation) can receive a short course or HBIG-free regimens but need continued monoprophylaxis with a potent NA. Conversely, patients with a high risk of recurrence (HBV DNA positive at liver transplantation, HDV coinfection, or poor adherence to NA therapy) should receive lifelong combination of HBIG and a potent NA [428-430].
Since a HBsAg-negative/anti-HBc-positive donor liver is suggestive of occult HBV infection, it is appropriate to preferentially transplant it to a HBsAg-positive recipient considering the possibility HBV reactivation after liver transplantation. HBsAg-negative patients receiving anti-HBc-positive liver grafts have different HBV reactivation rates from 10–80% depending on recipient’s immunization against HBV [431]. The HBV incidence rate was highest at 47.8% when the recipient was not immune to HBV (anti-HBs-negative, anti-HBc-negative) and lowest at 1.4% when both anti-HBs and anti-HBc were positive [432]. The HBV incidence rate was 13.1% in recipients with anti-HBs-negative and anti-HBc-positive, and 9.7% in recipients who developed immunity through HBV vaccine (anti-HBs-positive, anti-HBc-negative). In HBsAg-negative patients receiving liver transplantation from an anti-HBc-positive donor, administration of prophylactic antiviral drugs significantly reduced the incidence of HBV infection from 47.8% to 12% in recipients who were not immune to hepatitis B (anti-HBs-negative, anti-HBc-negative), from 15.2% to 3.4% in those with anti-HBc-positivity, and from 9.7% to 0% in those who developed immunity through HBV vaccine (anti-HBs-positive, anti-HBc-negative) [431]. Thus, treatment of patients receiving anti-HBc-positive liver grafts should depend on their immunization against HBV (Fig. 6). NAs should be started immediately after surgery and be continued. Further studies are needed to determine whether antiviral therapy is unnecessary in patients who are both anti-HBs and anti-HBc-positive. Lamivudine has been used mostly because of its favorable cost-effectiveness [433,434]; however, drugs with high potency and low rate of resistance such as entecavir, tenofovir DF, and tenofovir AF are expected to be used more in the future.

Strategies after liver transplantation in patients receiving anti-HBc-positive liver graft. Anti-HBc, antibody to HBcAg; HBsAg, hepatitis B surface antigen; NA, nucleos(t)ide analogue; HBIG, hepatitis B immunoglobulin; anti-HBs, antibody to HBsAg; HBV, hepatitis B virus. *Pooled data from the study by Cholongitas et al [431].
[Recommendations]
1. In HBV-related liver transplant recipients, prophylactic combination of HBIG and a potent NA posttransplantation is recommended for the prevention of HBV recurrence (B1).
- Patients with a low risk of recurrence (HBV DNA negative at liver transplantation) can receive a short course or HBIG-free regimens but need continued monoprophylaxis with a potent NA (B1).
- Patients with a high risk of recurrence (HBV DNA positive at liver transplantation, HDV coinfection, or poor adherence to NA therapy) should receive lifelong combination of HBIG and a potent NA (B1).
2. Considering the risk of renal dysfunction and/or bone diseases after liver transplantation, potent antivirals with low rate of resistance such as entecavir and tenofovir (AF or DF) are preferred antiviral drugs (B1), and besifovir can be considered (C1).
3. HBsAg-negative patients receiving anti-HBc-positive liver grafts show variation in HBV reactivation rate depending on the recipient’s immunization status against HBV (Fig. 6) and should receive NA therapy accordingly (B1).
Non-liver solid organ transplant recipients
All non-liver solid organ transplantation recipients should be evaluated for HBV infection and immunity with HBsAg, anti-HBc, and anti-HBs. Patients who are HBsAg-positive should undergo ALT and HBV DNA measurements and staging with biopsy or non-invasive fibrosis tests to determine whether advanced fibrosis or cirrhosis is present. HBsAg-positive renal transplant recipients are at high risk for persistent viral activity or reactivation and have a significantly higher mortality rate due to liver-related complications such as liver cirrhosis and HCC [435,436]. Recent reports indicate that antiviral therapy significantly decreased the HBV reactivation rate and increased the survival of HBsAg-positive renal transplant recipients [437,438]. Therefore, all HBsAg-positive organ transplant recipients should receive antiviral therapy before or right after liver transplantation. Entecavir and tenofovir (AF or DF) can be considered because of high potency and the low rate of resistance in long-term use, and patients who are at risk for renal dysfunction should receive entecavir and tenofovir AF preferably. With the introduction of effective antiviral therapy, patients with CHB and those with compensated cirrhosis without portal hypertension became able to receive solid organ transplants, and successful cases of kidney transplantation have been reported [439]. However, in patients with advanced or decompensated cirrhosis who are at risk of liver failure, simultaneous transplantation of the liver and other organs, such as kidneys, heart, and lungs, should be considered.
Recurrence of HBV in HBsAg-negative solid organ transplant recipients can be divided into two cases: one with transmission of HBV from a HBsAg-negative and anti-HBc-positive donor and two with reactivation in an anti-HBs-negative and anti-HBc-positive recipient after transplantation. The rate of HBsAg positivity after kidney transplantation caused by HBV transmission from HBsAg-negative and anti-HBc-positive kidney donor was very low at 0% to 3% [440-442]. The HBV reactivation rate in anti-HBs-negative and anti-HBc-positive kidney recipients varied from 0% to 12% depending on the recipient’s immunization against HBV [443-453]. The reactivation rate was significantly higher in recipients with antiHBc-positive and anti-HBs-negative (5.6–12.0%) than in those with anti-HBc-positive and anti-HBs positive (1.1–2.7%) [443,452,453]. Thus, HBsAg-negative, anti-HBc-positive recipients should be monitored for HBsAg positivity after solid organ transplantation, and NA of entecavir, tenofovir DF, or tenofovir AF should be administered in case of HBsAg detection.
[Recommendations]
1. All non-liver solid organ transplantation recipients should be tested for HBsAg, anti-HBc, and anti-HBs before transplantation (A1).
2. All HBsAg-positive or HBV DNA-positive solid organ transplant recipients should start prophylactic antiviral treatment at the time of transplantation (A1). Considering the risk of renal dysfunction and/or bone diseases after transplantation, potent antivirals with low rate of resistance such as entecavir and tenofovir (AF or DF) are preferred (B1), and besifovir can be considered (C1).
3. HBsAg-negative, HBV DNA-undetectable, but anti-HBc-positive solid organ transplant recipients should be monitored for HBV infection after transplantation (B1).
Coinfection with other viruses
Hepatitis C virus (HCV) coinfection
In patients with CHB, the anti-HCV-positivity rate varies from 1.5% to 2.4% in Korea [454,455]. Patients with HBV/HCV coinfection have more severe necroinflammation and fibrosis as well as high risk of cirrhosis and HCC compared to those with a monoinfection [456-459]. Patients with HBV/HCV coinfection should be tested for HBV DNA and HCV RNA before antiviral treatment for each virus. If HCV RNA is detectable, antiviral treatment for HCV (direct acting antiviral [DAA] therapy) should be started. If HBV DNA is detectable, check for ALT, HBV DNA, and presence of liver cirrhosis and treat according to the HBV guidelines, as with HBV monoinfection (Fig. 3).
There is a potential risk of HBV reactivation during DAA therapy or after clearance of HCV. In a meta-analysis, HBV DNA was newly detected or the level increased in 14.1% of patients after administration of DAAs for 4–12 weeks, whereas active hepatitis accompanied by ALT elevation was noted in 12.2% of patients [460]. Therefore, patients with HBV/HCV coinfection should be monitored for the risk of HBV reactivation by monitoring ALT and HBV DNA level during and after DAA therapy. In addition, if HBV is indicated for treatment at the time of starting DAA therapy for HCV, NA therapy for HBV should be started. In patients with a history of cirrhosis or HCC, simultaneous NA therapy for HBV can be considered along with DAA therapy for HCV to reduce the risk of liver failure risk resulting from HBV reactivation [461,462]. Entecavir, tenofovir DF, and tenofovir AF are preferred choices. There was no significant drug-drug interaction between NAs for HBV (entecavir, tenofovir DF, tenofovir AF) and DAAs or HCV. However, when selecting antiviral drugs in patients with HBV/HCV/HIV triple infection, drug-drug interactions should be considered [463,464].
In HBsAg-negative and anti-HBc-positive patients with HCV, HBV reactivation rate (HBsAg positivity) during DAA therapy is very low at 0–0.1% [465-467]. Although HBV reactivation rate during DAA therapy is low, HBsAg and HBV DNA should be tested to check for HBV reactivation in cases of ALT elevation.
[Recommendations]
1. If HCV RNA is detectable in patients with HBV/HCV coinfection, antiviral treatment for HCV should be started (A1).
2. If antiviral treatment for HBV is indicated in HBV/HCV coinfection, antiviral treatment for HBV should be started (Fig. 3) (A1).
3. Patients with HBV/HCV coinfection should be monitored for the risk of HBV reactivation by monitoring ALT and HBV DNA levels during and after DAA therapy (B1).
4. HBsAg-negative and anti-HBc-positive patients with HCV infection are at very low risk of reactivation with HCV-DAA therapy; however, HBsAg and HBV DNA should be tested to check for HBV reactivation in cases of ALT elevation (B1).
HDV coinfection
HDV is a defective RNA virus that does not encode its own envelope proteins, enters the liver through HBsAg-expressing hepatocytes, and replicates the HDV genome to express HDV antigens [468]. Thus, HDV infection occurs when people become infected with both HBV and HDV simultaneously (co-infection) or in CHB patients newly infected with HDV (super-infection). An estimated 12 million people worldwide have experienced HDV infection, and approximately 4.5% of HBV patients are estimated to have HDV [469]. In one Korean study, the HDV coinfection rate was 0.3% in 940 patients with CHB, including 75 patients with HCC [470]. In another study with 194 patients including 64 CHB patients and 130 HCC patients, the HDV infection rate was 3.6% [471]. Compared to HBV monoinfection, HBV/HDV coinfection is associated with higher rates of cirrhosis and HCC [472-474].
HDV infection can be diagnosed by detecting anti-HDV or HDV RNA in the serum or by detecting HDV antigens in liver tissues using immunohistochemistry. In patients with HDV infection, degree of hepatic fibrosis should be evaluated by liver biopsy or non-invasive methods irrelevant to HDV RNA detection. In HBV/HDV coinfected patients having advanced fibrosis or cirrhosis or patients who are indicated for antiviral treatment of CHB, NA therapy should be administered to prevent the progression of liver disease. However, NA therapy is not recommended in cases where HBV treatment is not indicated because NAs do not inhibit HDV replication.
All patients with HDV RNA positivity are eligible for treatment regardless of the degree of liver disease, and the primary endpoint of treatment is the suppression of HDV replication, which is usually accompanied by normalization of ALT level and improvement of necroinflammation on liver biopsy. The current standard treatment for HDV is weekly peginterferon alpha by subcutaneous injection for 48 weeks, and a sustained viral response at 24 weeks has been reported in 23–28% of patients [475,476]. A sustained virological response can be predicted by measuring serum HDV RNA level after 24 weeks of peginterferon alpha therapy [477]. However, relapse is frequent during long-term follow-up, as seen in one study with an average follow-up of 4.3 years where sustained virological response was maintained at only 12% [478]. Combination therapy using NAs and peginterferon alfa did not improve virological response compared to peginterferon alfa monotherapy [479]. In a small study using extended peginterferon alfa therapy for 24 months, 47% of patients achieved sustained virological response during an average follow-up of 19.5 months after treatment, but further studies are needed [480].
Because of the low efficacy of peginterferon alpha, new therapeutic antiviral agents for HDV are under development. Bulevirtide (BLV), a drug in phase III study, is an entry inhibitor for HDV. It is a lipopeptide that competitively acts on sodium taurocholate cotransporting polypeptide (NTCP), an entry receptor shared by HBV and HDV viruses [481]. In 2020, BLV was first approved as an antiviral agent for HDV under conditional authorization by the European Medical Agency for patients with compensated liver disease and positive HDV RNA with or without NAs. BLV 2 mg is administered subcutaneously daily and can be maintained as long as the virologic response persists. According to interim analysis of the ongoing phase 3 study, BLV treatment for 24 weeks resulted in significant decrease in HDV RNA and ALT. Virologic response (undetectable HDV RNA or ≥2 log10 IU/mL decrease from baseline) in patients with no treatment, BLV 2 mg, and BLV 10 mg was 4%, 55%, and 68%, respectively. Combined response (undetectable HDV RNA or ≥2 log10 IU/mL decrease from baseline and normal ALT) rate in patients with no treatment, BLV 2 mg, and BLV 10 mg was 0%, 37%, and 28%, respectively [482].
Another drug in phase III study is lonafarnib, an oral prenylation inhibitor that reduces HDV virus load by blocking prenylation, a process involved in virus assembly, replication, and subsequent hepatocyte infectivity [483]. Combination with ritonavir is known to increase by 4–5-fold in systemic exposure and to decrease gastrointestinal side effects. Virologic responses (undetectable HDV RNA or ≥2 log10 IU/mL decrease from baseline) at the end of treatment were reached in 46% and 89% of patients receiving the all-oral regimen of lonafarnib 50 mg bid + ritonavir, and combination regimen of lonafarnib (25 or 50 mg bid) + ritonavir + peginterferon alpha, respectively [484].
Antiviral agents for HDV under phase II study include peginterferon lambda, REP 2139 (nucleic acid polymers), and JNJ-3989 (RNAi) [485,486].
[Recommendations]
1. In CHB patients with low HBV DNA and elevated ALT levels, HDV screening with anti-HDV test can be considered, unless there is another suspected cause of elevated liver enzyme (C1).
2. In CHB patients with HDV coinfection having advanced fibrosis or cirrhosis, or patients who are indicated for antiviral treatment of CHB, NA therapy should be administered to prevent the progression of liver disease (B1).
3. CHB patients with HDV coinfection are recommended to be treated with peginterferon alfa for at least 1 year (A2), and administration of a new therapeutic agent can be considered according to the clinical course of the patient (C1).
Notes
Authors’ contributions
Jang JW, directed the guideline committee, and outlined and supervised the manuscript writing and editing; Choi J and An J edited the manuscript. All the committee members participated drafting the manuscript; Jang JW (introduction), Kang W (natural history), An J (emerging markers of HBV infection, and patients with HCC), Kwon JH (treatment indication), Kim TH (new drugs for a functional cure), Park JY (cessation of treatment and monitoring after antiviral treatment), Choi J (patients with renal dysfunction or metabolic bone disease and supplementary material), Yu SJ (patients on immunosuppression or chemotherapy, and liver transplant patients), and Chon YE (liver transplant patient, nonliver organ transplant recipients, and coinfection with other viruses) contributed to the manuscript writing.
Conflicts of Interest
Jang JW: Received honoraria from Gilead, Abbvie, Bayer, Roche, Sysmex, Ipsen. Received grants from Sysmex, Gilead, Yuhan, Dong-A ST, Hanmi
Park JY: Received grants from Gilead, Pfizer, MSD, BMS, Roche, Astrazeneca, Aramchol, Novonorodisk, Yuhan, Hanmi
Kwon JH: Received honoraria and grants from Gilead, Abbvie
Yu SJ: Received honoraria and grants from Gilead, Abbvie, BMS, Daewoong, Yuhan, Chongkundang Pharm, Dong-A ST, Samil, Samjin
Kang W: Received honoraria and grants from Gilead, BMS, Abbvie, Eisai, Bayer, Roche, MSD, Yuhan, Dong-A ST, Ildong, Daewoong, Samil, Boryung
Chon YE: Received honoraria from BMS, Dong-A ST, Abbvie, Daewoong
Kim TH: Nothing to declare
Choi J: Received honoraria from Gilead, Abbvie, Daewoong
An J: Received honoraria from Yuhan
Abbreviations
ACLF
acute-on-chronic liver failure
AF
alafenamide fumarate
ALT
alanine aminotransferase
anti-HBe
antibody to HBeAg
anti-HBs
antibody to HBsAg
anti-HBc
antibody to HBcAg
APRI
aspartate aminotransferase-to-platelet ratio index
ASO
antisense oligonucleotides
BCP
basal core promoter
BLV
bulevirtide
CAGE-B
cirrhosis and age
CAR
chimeric antigen receptor
cccDNA
covalently closed circular DNA
CHB
chronic hepatitis B
CI
confidence interval
DAA
direct acting antiviral
DF
disoproxil fumarate
DMARDs
disease-modifying antirheumatic drugs
EBRT
external beam radiation therapy
eGFR
estimated glomerular filtration rate
EOT
end-of-treatment
FIB-4
fibrosis-4
GRADE
Grading of Recommendations, Assessment, Development, and Evaluation
HBcAg
hepatitis B core antigen
HBcrAg
hepatitis B core-related antigen
HBeAg
hepatitis B e antigen
HBIG
hepatitis B immunoglobulin
HBsAg
hepatitis B surface antigen
HBV
hepatitis B virus
HCC
hepatocellular carcinoma
HCV
hepatitis C virus
HDV
hepatitis D virus
HIV
human immunodeficiency virus
HR
hazard ratio
HSCT
hematopoietic stem-cell transplantation
IBD
inflammatory bowel disease
KASL
Korean Association for the Study of the Liver
MELD
Model for End-stage Liver Disease
NA
nucleos(t)ide analogue
NTCP
sodium taurocholate cotransporting polypeptide
OR
odds ratio
p22Cr
p22 corerelated antigen
PAGE-B
platelets, age, gender, and hepatitis B scores
PAMPs
pathogen-associated molecular patterns
PC
precore
pgRNA
pregenomic RNA
R-CHOP
rituximab-CHOP
RA
rheumatoid arthritis
RCT
randomized controlled trial
REACH-B
risk estimation for HCC in chronic hepatitis B
RIG
retinoic acidinducible gene
RISC
RNA-induced silencing complex
RNAi
RNA interference
RR
relative risk
SAGE-B
stiffness and age
siRNA
small interfering RNA
SVPs
subviral particles
TACE
transarterial chemoembolization
TE
transient elastography
THRI
Toronto HCC risk index
TLR
toll-like receptor
TNFα
tumor necrosis factor-alpha
ULN
upper limit of normal