Unmet needs of chronic hepatitis C in the era of direct-acting antiviral therapy
Article information
Abstract
The treatment of chronic hepatitis C (CHC) has been revolutionized in an era of all-oral direct-acting antivirals (DAAs) since 2014. Satisfactory treatment efficacy and tolerability can be provided by novel DAAs. Nevertheless, there are still some unmet needs and emerging issues in the treatment of CHC in the DAA era. Certain hard-to-cure populations are prone to have inferior treatment responses, including patients with severe liver decompensation, active hepatocellular carcinoma (HCC), and hepatitis C virus (HCV) genotype 3 (HCV-3) infection and those who experience multiple DAA treatment failures. Hepatitis B virus (HBV) reactivation during and after DAA treatment has raised concern regarding the use of prophylactic antivirals against HBV throughout DAA treatment. However, the standard strategy for the use of prophylactic antivirals is not uniform across regional guidelines. In the post-sustained virological response (SVR) period, HCC still occurs in a substantial proportion of patients. Due to the relatively short follow-up period, the net benefit of the achievement of an SVR by DAAs in the reduction of extrahepatic manifestations has not yet been determined. Attention must also be paid to HCV reinfection, particularly in high-risk populations. The most critical and unmet need for HCV elimination is the large gap in the HCV care cascade at the population level. To accomplish the World Health Organization (WHO)’s goal for HCV elimination by 2030, the expansion of access to HCV care requires a continuous effort to overcome practical and political challenges.
INTRODUCTION
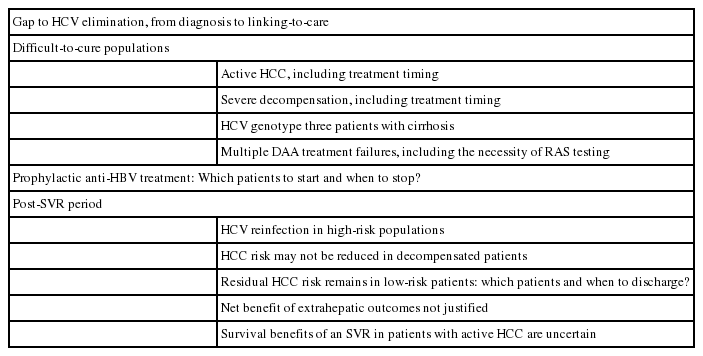
The treatment of chronic hepatitis C (CHC) has been revolutionized in an era of all-oral direct-acting antivirals (DAAs) since 2014. With the use of current novel DAAs, a sustained virological response (SVR) rate >95% can be attained in addition to satisfactory tolerability. Nevertheless, there are still some unmet needs and emerging issues in the treatment of CHC in the DAA era. Inferior treatment efficacies are observed in some hard-to-cure populations, including patients with severe liver decompensation, active hepatocellular carcinoma, hepatitis C virus (HCV) genotype 3 (HCV-3) infection and those who experience multiple DAA failures. Hepatitis B virus (HBV) reactivation during and after DAA treatment has raised concern for the use of prophylactic antivirals against HBV throughout the course of DAA treatment. However, there is no definite recommendation to guide which patients should be prescribed these prophylactic antivirals and for how long. In the post-SVR period, hepatocellular carcinoma (HCC) still occurs in a substantial proportion of patients, especially among those with advanced fibrosis and subjects who possess ongoing risk factors (e.g., diabetes, HBV dual infection, alcoholism). Due to the relatively short follow-up period, the net benefit of the achievement of an SVR by DAAs in the reduction of extrahepatic manifestations has not yet been settled. Attention must also be paid to HCV reinfection, particularly in high-risk populations. Last but not least, the most critical issue for HCV elimination is the large gap in the HCV care cascade at the population level. The abovementioned issues will be highlighted and discussed in the current review (Table 1).
DIFFICULT-TO-CURE POPULATIONS
HCV-3
HCV-1 is considered a difficult-to-cure genotype in the interferon era. In the landscape of the DAA era, the treatment response of HCV-1 infection is no longer suboptimal. Rather, a relatively lower SVR rate could be observed in the treatment of HCV-3 infection, particularly in patients with liver cirrhosis who failed prior antivirals. Ironically, patients with HCV-3 infection are prone to have advanced liver disease at the time of presentation [1]. In the ASTRAL-3 study, a high SVR12, defined as undetectable HCV RNA throughout 12 weeks of the posttreatment follow-up period, of 95% could be attained in HCV-3 infected patients treated with sofosbuvir (SOF) plus velpatasvir (VEL). However, the SVR rate was only 89% in the subgroup of treatment-experienced patients with cirrhosis [2]. While the pooling analysis of phase III studies of SOF/VEL revealed an SVR rate of 93% in patients with nonstructural protein 5A (NS5A) resistance-associated substitutions (RASs) [3], a lower SVR rate of 84% was denoted in patients with the Y93H mutation in the ASTRAL-3 study [2]. This finding may prompt RAS testing or the addition of ribavirin to the regional guidelines for the treatment of patients with cirrhosis with HCV-3 infection receiving SOF/VEL [4-6]. Beyond the impact of RASs, HCV-3 subtyping may also account for treatment inferiority. In a phase III study using SOF/VEL for 12 weeks in Asia, an SVR rate of only 76% was noted in HCV-3b-infected patients. Among them, an SVR rate of 89% in patients without cirrhosis but only 50% in patients with cirrhosis patients was depicted [7]. For the other pangenotypic DAA regimen, glecaprevir (GLE)/pibrentasvir (PIB), an SVR rate of 94.9% could be attained in HCV-3 treatment-naïve patients without cirrhosis who received 8 weeks of treatment in the ENDURANCE-3 study. Among them, the SVR rate of patients with the A30K mutation in NS5A was 75% (12/16) compared to 99% (135/137) in those without the mutation [8]. As HCV-3 treatment-naïve compensated patients with cirrhosis could be allocated to abbreviated 8-week GLE/PIB regimen with an SVR rate of 98.4% (60/61) in the per-protocol analysis of the EXPEDITION-8 study [9], interferon/ribavirin- or SOF (PRS)-experienced patients should remain on a 12- to 16-week regimen of GLE/PIB to ensure treatment efficacy [4,5].
Liver decompensation
The portal-systemic-shunting-related poor first-pass effect and bioavailability of DAAs may result in the suboptimal antiviral response in patients with liver decompensation [10]. SOF plus NS5A inhibitor-based therapy is recommended since protease inhibitors are contraindicated. In the SOLAR-1 and SOLAR-2 studies, the SVR12 rate was 87% and 85–86% in Child-Pugh class B and Child-Pugh class C patients after 12 weeks of SOF/ledipasvir (LDV) treatment, respectively [11,12]. In the ASTRAL-4 study, although the treatment efficacy could be improved up to 94% in Child-Pugh class B patients who were allocated to 12 weeks of SOF/VEL plus ribavirin treatment, the SVR12 rate was as low as 85% in patients with HCV-3-infection [13]. Adding ribavirin to DAAs in the population is warranted to ascertain treatment efficacy. The role of ribavirin in the improvement in DAA efficacy is not fully understood [14], and whether it improves early kinetics and promotes immune modulations to diminish the chance of relapse following viral mutation awaits further identification [15]. It should be noted that the failure to attain an SVR in a significant proportion of decompensated patients was due to adverse events or mortality-related treatment discontinuation rather than to virological failure in the trials [11-13], which reflected the difficulty in the management of the fragile subjects in the clinical setting. For example, a recent study using SOF/VEL plus ribavirin in the treatment of 23 patients with Child-Pugh class C resulted in an SVR12 of only 70% (16/23) in an intention-to-treat analysis. The lack of assessment in six of the seven patients during the study period was due to mortality not related to the study drugs [16].
Apart from the issue of treatment efficacy, ongoing deterioration of the Model for End-Stage Liver Disease (MELD) score was noted in 17–43% of Child-Pugh class B and 11–18% of Child-Pugh class C patients, even after the eradication of HCV [17]. The timing of DAA initiation before or after liver transplantation in decompensated patients on the waiting list increases the complexity when donor feasibility is taken into account. It is recommended that patients whose MELD score is >18–20 should be considered for liver transplantation first if the local situation allows and if the donor is available within 6 months [4,18]. On the other hand, although patients could be delisted due to an improvement in liver function reserve by DAAs before liver transplantation, this may occur in only a limited subset of patients [19]. Furthermore, re-decompensation [20], a loss of transplantation priority with the occurrence of HCC, and a poor quality of life (so-called MELD purgatory) [21] become challenging tasks clinicians must face in the postSVR period.
Multiple DAA treatment failures
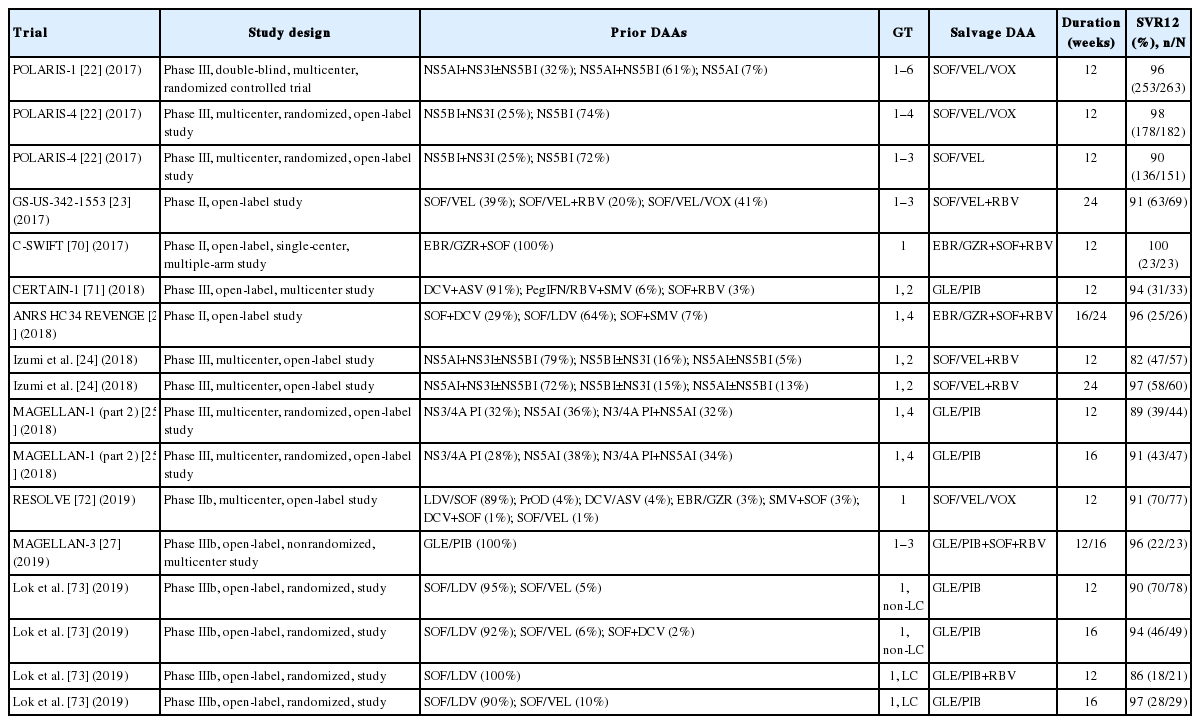
For patients with previous DAA treatment failures, 12-week SOF/VEL/voxilaprevir (VOX) and 24-week SOF/VEL plus ribavirin treatment for patients with HCV-1-6 infection have been approved [22-24]. For HCV-1 infected patients, 12-week GLE/PIB treatment for prior NS3/4A protease inhibitor-experienced patients and 16-week GLE/PIB treatment for prior NS5A inhibitor-experienced patients have also been approved [25]. Recently, 179 DAA-experienced patients were treated with SOF/VEL/VOX with or without RBV in the real-world setting. Of them, 82% of patients carried a RAS in the NS3, NS5A or NS5B regions before retreatment. The overall SVR12 rate was 96% in a per-protocol analysis. Nevertheless, patients with liver cirrhosis (91%, 71/78) and HCC (71%, 5/7) had a significantly lower SVR rate. Due to overlapping antiviral classes, a relatively low SVR rate of 88.4% (23/26) was noted in SOF/VEL-experienced patients who received SOF/VEL/VOX [26], indicating that the retreatment of prior-DAA-failed patients should be managed sophisticatedly and on an individual basis.
Certain unapproved strategies with multitarget regions have also been adopted with satisfactory efficacy [27,28]. The timing and necessity of RAS testing remain elusive [4,5]. Currently, the treatment strategy for “very difficult-to-cure” patients, who have been defined as patients with NS5A RASs who failed twice to achieve an SVR after a combination regimen including a protease inhibitor and/or an NS5A inhibitor, remains unclear. The European Association for the Study of the Liver (EASL) guideline has advocated the use of SOF/VEL/VOX or SOF/GLE/PIB in addition to ribavirin and has extended the treatment duration to 16–24 weeks. Nevertheless, the recommendation awaits validation by prospective studies (Table 2).
Finally, since protease inhibitors are contraindicated for decompensated patients, the only recommended regimen is the combination of SOF/VEL+RBV for 24 weeks. However, the SVR rate might be suboptimal in patients with baseline NS5A RASs and those who could not tolerate full course ribavirin. This highlights the urgent need for early identification and treatment for CHC patients to avoid progression of liver disease to decompensated cirrhosis [29].
Patients with active HCC
Treatment efficacy in patients with HCC has been widely discussed (Fig. 1). Compared to patients without HCC or with inactive HCC, whether patients with active HCC have an inferior treatment response remains controversial [10,30]. A recent meta-analysis of 49 studies showed that the SVR rate was significantly lower in patients with active HCC (73.1%) than in those with inactive HCC (92.6%) or without HCC (93.3%) [31]. The argument exists that in these studies, there were unequal patient and viral characteristics as well as suboptimal regimens in early studies, which may end in different treatment responses between groups. To overcome this pitfall, Ogawa et al. [32] conducted a propensity-score-matched study for age, sex, cirrhosis, prior treatment, HCV genotype, treatment regimen, baseline platelet count, HCV RNA, total bilirubin, alanine aminotransferase, and albumin level to evaluate the treatment outcome in a large Asian cohort with or without HCC. Patients with active HCC (85.5%) but not those with inactive HCC (93.7%) had a lower SVR rate than those without HCC (95.0%; adjusted odds ratio, 0.28; P=0.01).
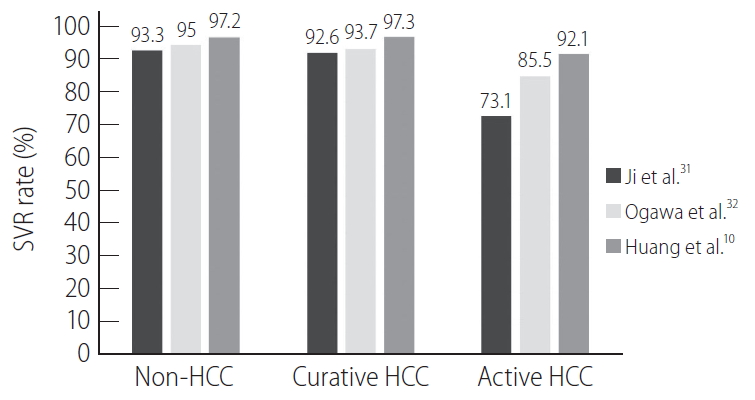
Treatment responses of chronic hepatitis C patients with different hepatocellular carcinoma status across studies. SVR, sustained virological response; HCC, hepatocellular carcinoma.
A significantly improved survival benefit has been observed in early-stage HCC patients who received curative cancer treatment followed by DAA therapy [33]. If liver function does not compromise cancer treatment (e.g., as in the case of the ability to undergo anesthesia for surgical resection), HCC with curative potential should be treated first before antiviral therapy to ensure a higher chance of treatment success. On the other hand, the timing of the initiation of DAAs in patients with incurable HCC becomes a challenging issue since residual HCC may compromise DAA treatment efficacy. Patients with active HCC may have more safety concerns during DAA treatment [10]. In addition, with the breakthrough innovations of systemic therapy in advanced HCC, whether the treatment with DAAs and immunotherapy/target therapy alter the clinical outcome remains unknown [34]. Furthermore, the most critical point is whether HCV eradication will prolong long-term survival in patients with incurable HCC. Dang et al. [35] conducted a propensity-matched study enrolling 1,239 untreated patients and 437 patients with an SVR. Among them, 70.6% of patients received curative HCC treatment, whereas the remaining 29.4% received palliative treatment. The results demonstrated that the attainment of an SVR significantly reduced 5-year all-cause mortality and liver relative mortality in both groups [35], indicating that treatment should not be withheld from those who are not eligible for curative HCC therapy. One of the postulated reasons is that the recovery or preservation of liver function after HCV eradication may offer chances for repeated cancer therapy. Nevertheless, patient numbers in certain subpopulations were too limited for the conclusions to be generalized. Upon closer look, patients with Child-Pugh class B did not benefit much from HCV eradication in terms of the long-term outcome, whereas the achievement of an SVR did not provide survival benefits in patients with Child-Pugh class C [35]. Unlike decompensated patients without HCC, the deteriorated liver function in HCC patients may be due to cancer burden, which also precludes them from liver transplantation. The role of HCV eradication in the terminally ill population remains an unknown domain for exploration.
HBV REACTIVATION IN PATIENTS WITH DUAL HCV/HBV INFECTION
The issue of HBV reactivation after HCV viral suppression deserves more attention, particularly in HBV hyperendemic areas [36-38]. A meta-analysis of 17 studies observed that the rate of HBV activation was 24% in chronic hepatitis B patients, which in turn led to 9% clinical hepatitis. A total of 1.4% of patients with resolved HBV experienced HBV reactivation, but none had clinical relapse [39]. Until now, no prospective controlled study has denoted which, when, or for how long HBV patients should receive prophylactic nucleoside/nucleotide analogs (NUCs) or monitoring during/post-DAA therapy. The recommendations of regional guidelines are largely based on clinical rationality, and the level of evidence is not strong enough to draw conclusions. In addition, there are somewhat different viewpoints among regional guidelines [4,5,40]. For example, the EASL suggests that patients who are hepatitis B virus surface antigen (HBsAg)-positive should receive HBV prophylaxis at least until week 12 post-DAA therapy and be monitored monthly if HBV treatment is stopped [4]. The Asian Pacific Association for the Study of the Liver (APASL) suggests pre-emptive NUC prophylaxis in HBV patients with advanced liver disease or pre-existing HCC. For patients with mild liver disease, close monitoring without anti-HBV therapy is another option. When to stop NUCs should follow APASL HBV guidelines [40]. One of the best ways to monitor these patients is to identify a surrogate marker that can positively and negatively predict HBV reactivation. Factors predictive of HBV flares have been detectable HBV DNA before DAA therapy [39], alanine aminotransferase >2 times the upper limit of normal at baseline [37], and HBsAg >10 IU/mL [41] at baseline when using DAAs or baseline HBV DNA >300 IU/mL when using interferon-based therapy [38]. However, the surrogates were just identified as statistically significant in distinguishing events between groups, and their accuracies in guiding clinical decisions need to be validated by prospective studies. HBV-related clinical hepatitis may lead to severe decompensation and even mortality [41]. From this viewpoint, patients with advanced liver fibrosis should at least receive HBV prophylaxis before and during DAA therapy. The treatment duration and follow-up strategy after the discontinuation of NUCs awaits further clarification.
POST-SVR ERA
HCC
Similar to interferon-based therapy [42], the achievement of an SVR by DAAs decreases the risk of HCC occurrence and does not increase the risk of HCC recurrence [43,44]. Notably, the benefits of the achievement of an SVR in the reduction of HCC risk in decompensated patients are controversial [20,45]. Again, this controversy raises the importance of the discussion of the ideal timing for liver transplantation in the treatment of decompensated patients mentioned earlier. Several factors have been predictive of HCC in the post-SVR period [46-50]. The developed prediction model for HCC has also been created to guide follow-up strategies [51,52]. It is noteworthy that the risk of HCC persists even after 10 years of viral eradication [53]. It has been suggested that HCV-induced oncogenic effects are elicited before treatment and that the “epigenetic scar” may leave and persist long after viral eradication, leading to a life-long risk of HCC [54]. The EASL advocates that patients with an SVR could be discharged if they do not possess advanced fibrosis or other comorbidities. The recommendation may be based on the cost-effectiveness of surveillance. Nevertheless, HCC still occurs in so-called low-risk patients after viral eradication [49]. As the updated APASL guidelines recommended the follow-up of patients with an SVR at different intervals based on their underlying risks [40], the surveillance of HCC, particularly low-risk patients, in the post-SVR period should be judged on a case-by-case basis including local medical accessibility and feasibility [55].
Extrahepatic manifestations
HCV eradication may also improve long-term extrahepatic manifestations. Due to the short follow-up period of the post-DAA period and low incidences of index outcomes, the benefits of DAAs in the reduction in extrahepatic complications are not universally granted [56]. A retrospective cohort study has shown that the risk of non-Hodgkin lymphoma was not significantly reduced after DAA-induced SVR (hazard ratio, 0.86; 95% confidence interval, 0.52–1.43) after only 2.01 years of follow-up [56]. HCV eradication may reduce the risk of vascular events [57]. However, this was not always the case. A study comprising 160,875 subjects showed that the risk of coronary heart disease and stroke did not differ between patients with and without an SVR [58]. Hypolipidemia during viremic status can be reversed to deteriorate lipid profiles after DAA therapy [59]. An increase in the small dense low-density lipoprotein cholesterol level corresponding to increased carotid intima-media thickness 1 year after DAA therapy has been reported [60]. Attention should be paid not only to liver-related complications but also to the extrahepatic consequence of cardio-cerebrovascular disease in the post-SVR period.
Reinfection
The reinfection rate after HCV eradication should be rare in the general population. Nevertheless, reinfection has been frequently encountered in patients with high-risk behaviors. A systemic review showed that the HCV reinfection rate 5 years after HCV eradication was 0.95% in a low-risk population, 10.7% in a high-risk population (prisoners and patients who inject drugs), and up to 15.0% in subjects with HCV and human immunodeficiency virus (HIV) coinfection [61]. Due to the ease of access and the fact that more treatment candidates are allowed in the DAA era, it is postulated that more HCV reinfection may occur [62]. An annual incidence of up to 5.9% per person-year has been reported in HCV/HIV co-infected men who have sex with men after DAA therapy [63]. Multidisciplinary approaches to high-risk populations should be adopted, which include health counseling for safe sex, harm reduction services, opioid substitution therapy and so forth. Treatment as prevention and an increase in the treatment uptake rate followed by a decrease in viral reservoirs at the population level are the most critical policies to be applied [64].
GAP TO ACHIEVE HCV ELIMINATION
The WHO has set ambitious goals for the control of viral hepatitis by 2030 [65]. However, only a few countries are on track for HCV elimination. In the DAA era, where treatment efficacy and tolerability are no longer the major concern, the identification of multiple barriers that exist in patients, providers and institutions that prevent the delivery of HCV care is the most difficult task [66]. A comprehensive HCV care cascade involves blood safety and infection control, harm reduction, proper screening for unawareness, accurate and efficient diagnosis, and linking to medical care. To scale up prevention and treatment, outreach screening programs would be the key determinant for HCV elimination [67]. Several outreach screening programs and treatment strategies with the concept of microelimination in hyperendemic areas and high-risk populations have been adopted in Taiwan [68,69].
CONCLUSIONS
By using multitarget DAA therapy with high genetic barriers, adding ribavirin and extending treatment duration, difficult-to-cure patients may no longer be difficult to cure. The long-term benefit of HCV eradication in decompensated and/or active HCC patients remains elusive. Since it is impractical to conduct a prospective untreated-comparator study, larger observational studies comprising diverse patient characteristics with a longer follow-up period are warranted to judge the impact of SVR. For subjects with HBV/HCV dual infection who do not fulfill the indications for anti-HBV therapy before DAA treatment, the addition of prophylactic NUCs is practical and essential. Further studies that explore the pathophysiological interaction of the two viruses may help to identify the candidates for and strategy of NUC prophylaxis. Much work remains to be done with many barriers standing in the way of achieving the WHO goal of HCV elimination. Each step may require the support and engagement of the local healthcare system, infrastructural commitment and nongovernment organizations. The expansion of access to HCV care requires a continuous effort to overcome the practical and political challenges.
Acknowledgements
The study was supported by grants from Kaohsiung Medical University (MOST 107-2314-B-037 -025 -MY2, MOST 108-2314-B-037-003) and Kaohsiung Medical University Hospital (MOHW108-TDU-B-212-133006).
Notes
Authors’ contributions
CFH and MLY contributed to the design and writing of the manuscript.
Conflicts of Interest
Ming-Lung Yu: Research support (grant) from AbbVie, Abbott, BMS, Gilead, and Merck. Consultant for AbbVie, Abbott, Ascletis, BMS, Gilead, Merck and PharmaEssentia. Speaker for AbbVie, Abbott, Ascletis, BMS, Gilead, and Merck.
Chung-Feng Huang: Speaker for AbbVie, Abbott, BMS, Gilead, and Merck.
Abbreviations
APASL
Asian Pacific Association for the Study of the Liver
CHC
chronic hepatitis C
DAAs
direct-acting antivirals
EASL
European Association for the Study of the Liver
GLE
glecaprevir
HBsAg
hepatitis B virus surface antigen
HBV
hepatitis B virus
HCC
hepatocellular carcinoma
HCV
hepatitis C virus
HIV
human immunodeficiency virus
LDV
ledipasvir
MELD
Model for End-Stage Liver Disease
NS5A
nonstructural protein 5A
NUC
nucleotide analog
NUCs
nucleos(t)ide analogs
PIB
pibrentasvir
RAS
resistance-associated substitutions
SOF
sofosbuvir
SVR
sustained virological response
VEL
velpatasvir
VOX
voxilaprevir
WHO
World Health Organization