Efficacy and safety of daclatasvir plus asunaprevir for Korean patients with HCV genotype Ib infection: a retrospective multi-institutional study
Article information
Abstract
Background/Aims
The combination of daclatasvir (DCV) and asunaprevir (ASV) has demonstrated a high sustained virologic response at 12 weeks (SVR12) and a low rate of adverse events in previous clinical studies. The purpose of this study was to clarify the results of treatment and side effects in Korean patients with chronic hepatitis C virus (HCV) genotype Ib infection.
Methods
We retrospectively analyzed clinical data from chronic HCV genotype Ib patients treated with DCV+ASV from August 2015 to September 2016 at five hospitals in the Daejeon-Chungcheong area.
Results
A total of 152 patients were examined for resistance associated variants (RAVs). Among them, 15 (9.9%) were positive for Y93 and one (0.7%) was positive for L31. Of 126 patients treated with DCV+ASV, 83 patients completed treatment and 76 patients were included in safety and efficacy analysis. Five (6.6%) were positive for Y93 and 12 (15.8%) exhibited cirrhotic change. DCV+ASV was the first-line treatment for 58 (76.3%) patients. Eleven (14.5%) patients relapsed after previous treatment that included interferon and seven (9.2%) of these patients were found to be intolerant of interferon. Adverse events occurred in 10 (13.2%) patients and two patients stopped the medication because of severe itching and skin rash. SVR12 was 89.5% (68/76) in all patients and 91.5% (65/71) in RAV-negative patients.
Conclusions
DCV+ASV showed good efficacy in patients with HCV Ib infection in Korea. Close monitoring is needed for severe adverse events and treatment failure, which were uncommon.
INTRODUCTION
Chronic hepatitis C virus (HCV) infection was once best treated using peginterferon plus ribavirin. The difficulties with this treatment included severe adverse events, inconvenience of injection, and the long treatment duration required. The cure rate was only 60-70%, although it has improved [1].
This treatment was supplanted by use of direct acting antivirals (DAAs) in the mid-1990s. The cure rate increased to over 90%, the treatment duration was shortened, and treatment became more convenient since an injection was not required. Peginterferon is no longer recommended as the first-line treatment [2-4].
The combination of daclatasvir (DCV) and asunaprevir (ASV) was developed in Japan for the treatment of HCV genotype Ib. A few clinical studies including some Korean patients reported the high sustained virologic response at 12 weeks (SVR12) rate and a low rate of adverse events. The DCV+ASV combination was the first DAA approved for use in Korea in August 2015 with some usage limitations. This study aimed to clarify the results of treatment and side effects in Korean patients with chronic HCV genotype Ib infection treated with DCV+ASV.
MATERIALS AND METHODS
Patients
We included the patients who was infected with HCV genotype Ib and took examination for resistance associated variants (RAV) at 5 university hospitals located on Daejeon-Chungcheong area. The patients with decompensated cirrhosis were excluded by calculating Child-Pugh score with abdominal sonography, computed tomography, and blood test. The enrolled patients were divided into the treatment naïve group, treatment failure group (including null response, partial response, virologic breakthrough, relapse to previous treatment), and intolerant group (discontinued peginterferon because of side effects). The definition of virologic response followed the Korean Association for the Study of the Liver (KASL) guideline [3]. Positive RAV rate was determined in all enrolled patients. Rates of end of treatment response (ETR), SVR12, adverse events were analyzed in patients who completed the treatment. The protocol was approved by the institutional review board or independent ethics committee at each site. Patients were not required to give informed consent because this study used clinical data obtained after patients had agreed to treatment before initiation of treatment.
RAV examination
RAV examination focused on variants of non-structural 5A (NS5A). All five sites submitted samples for testing at Seoul Medical Science Institute. NS5A was detected by direct sequencing using a Veriti 96 well thermal cycler and genetic analyzer (Applied Biosystems, Carlsbad, CA, USA). The limit of detection of HCV RNA was 5,000 IU/mL. The result was recorded as positive or negative according to presence of RAV sites L31 or Y93. If result was “not detected”, reexamination of HCV genotype was done to exclude the other genotype.
Clinical and laboratory assessments
Hemotological and biochemical tests were performed before and during treatment at as close as possible to 4, 8, 16, 24 weeks during and 12 weeks after treatment. Cirrhosis of the liver was diagnosed by abdominal sonography and computed tomography. The Child-Pugh score was calculated to determine the difference in treatment results. Adverse events were identified by review of medical records and were confirmed concerning onset time, severity, and duration by discussion with co-authors at each site.
Measurement of HCV RNA and assessment of treatment efficacy
HCV RNA was measured by a real-time PCR assay at each site. The lower limit of quantification (LLOQ) of HCV RNA was 15 IU/mL at four sites and 40 IU/mL at the other site. Virologic breakthrough was defined as an increase of HCV RNA >1 log10 from nadir or HCV RNA ≥LLOQ after a measurements below LLOQ. ETR was defined as undetectable HCV RNA at the end of 24 weeks of treatment and SVR12 was defined as undetectable HCV RNA at 12 weeks after treatment.
Statistical analyses
Data were expressed as mean±standard deviation. Chi-square test or Student’s t-test were for univariate analyses. P<0.05 was considered statistically significant.
RESULTS
Prevalence of RAV
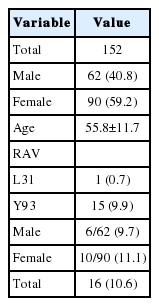
Total 152 patients comprised of 62 (40.8%) men and 90 (59.2%) women took RAV examination. Their mean age was 55.8±11.7 years old. Fifteen (9.9%) patients and one (0.7%) patient showed positive RAV at Y93 and L31, respectively. No patient showed positive RAV at both regions. Six (9.7%) men and 10 (11.1%) women showed positive RAV with no significant difference. The prevalence of RAV was 10.6% (Table 1).
Baseline characteristics of the treated patients
Of 126 patients treated with DCV+ASV, 83 patients finished the treatment. Seven patients were excluded due to follow-up loss (n=5), HCC recurrence (n=1) and expire because of accident (n=1). Finally, 76 patients including 32 (42.1%) men and 44 (57.9%) women were analyzed for safety and efficacy (Fig. 1). Mean age was 54.7±10.9 years. Five (6.6%) patients who showed positive RAV (Y93) were included. Compensated cirrhosis was present at baseline in 14 (18.4%) patients and absent in 62 (81.6%) patients. There were 58 (76.3%) treatment-naïve patients, 11 (14.5%) treatment failure patient s, and 7 (9.2%) patient s who did not tolerate peginterferon+ribavirin treatment (Table 2).

Flow chart summarizing patient selection. HCV, hepatitis C virus; NS5A, non-structural 5A; RAV, resistance-associated variant; DCV, daclatasvir; ASV, asunaprevir.
Adverse event
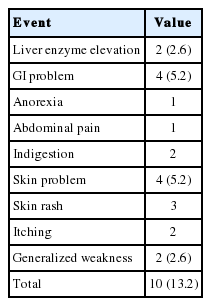
Adverse treatment-related events were reported in 10 (13.2%) patients. Transient aminotransferase increase <3-fold was noted in two (2.6%) patients. Four (5.2%) patients complained of gastrointestinal discomfort including anorexia (n=1), abdominal pain (n=1), and indigestion (n=2) without discontinuing treatment. Four (5.2%) patients suffered from skin rash and itching. Among them, two patients stopped the drugs due to severe itching. One of the two patients was a 59 year old female who relapsed after peginterferon+ribavirin treatment. She stopped treatment after 8 days. The second patient was an 80 year old female who had no previous treatment history. She first stopped the drugs at 20 days after treatment and restarted again after several days. But she finally stopped after 4 months because of recurrence of severe itching and rash. HCV RNA was not detected at 12 weeks after treatment (Table 3).
Virologic response
Seventy-six patients were analyzed for virologic response. SVR12 was 89.5% (68/76) overall, 89.7% (52/58) in the treatment-naïve group, 81.8% (9/11) in the treatment-failure group, and 100% (7/7) in the treatment-intolerant group. SVR12 for negative RAV patients was 91.5% (65/71) overall, 90.9% (50/55) in the treatment-naïve group, 88.9% (8/9) in the treatment-failure group, and 100% (7/7) in the treatment-intolerant group (Fig. 2).
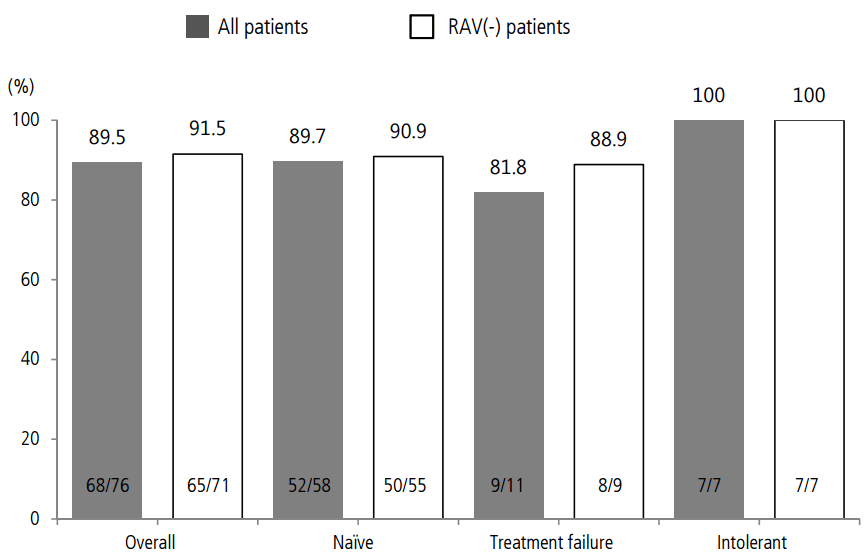
SVR12 overall and by prior treatment status in patients treated with DCV+ASV analyzed according to the presence of RAV. SVR12 was higher in RAV(-) patients than in RAV(+) patients. DCV, daclatasvir; ASV, asunaprevir; RAV, resistance-associated variant; SVR12, sustained virologic response 12 weeks post-treatment.
Sixty nine (90.8%) patients achieved ETR. The one patient displayed detectable RNA at 24 weeks after treatment. Y93 was positive on pretreatment examination of the patient. One patient who achieved ETR showed detectable HCV RNA at 12 weeks after cessation of treatment. The patient was relapsed after 48 weeks treatment of peginterferon+ribavirin and showed positive Y93.
SVR12 was analyzed according to baseline factors of age, sex, presence of cirrhosis, Child-Pugh score, and HCV RNA level. There was higher SVR12 tendency in patients younger than 60 years, in male, without cirrhosis, and HCV RNA level ≥6 log10 IU/mL without statistical significance (Fig. 3).
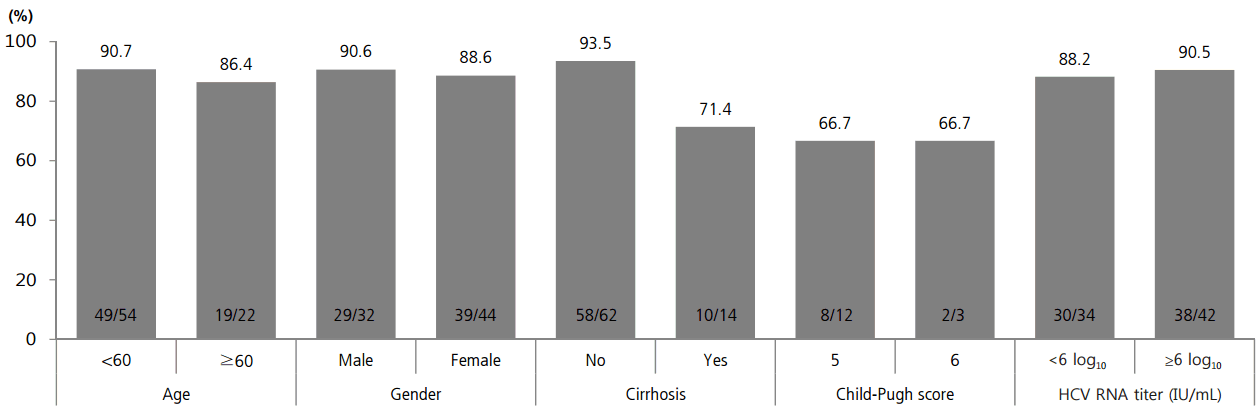
SVR12 according to baseline age, sex, presence of cirrhosis, Child-Pugh score, and pretreatment HCV RNA level. There was a higher SVR12 tendency in those who were male, younger than 60 years, without cirrhosis, and with an HCV RNA level ≥6 log10 IU/mL, but these findings were not statistically significant. HCV, hepatitis C virus; SVR12, sustained virologic response 12 weeks post-treatment.
DISCUSSION
DCV is a NS5A inhibitor that displays antiviral activity to all HCV genotypes in vitro [5]. ASV is a NS3 protease inhibitor that has antiviral activity to HCV genotypes 1, 4, 5, and 6 in vitro [6]. The authors of the study conducted in Japan reported that DCV+ASV produced high and low SVR to HCV genotype Ib and Ia, respectively. Use of DCV+ASV only for HCV genotype Ib in Japan was recommended and implemented. Insurance coverage of these drugs in Korea in effect since August 2015 limited against genotype Ib [7].
This genotype is the most prevalent subtype in Korea, accounting for a reported 45.4% of cases of HCV [8]. DCV+ASV have been widely prescribed for treatment of chronic HCV infection in Korea, given the aforementioned findings and insurance coverage. The higher SVR of DCV+ASV compared with peginterferon+ribavirin is an advantage. Use of DCV+ASV produced SVR12 in 83.7% of treated Asian patients in a clinical study [9]. Subgroup analysis in the same study revealed SVR12 rates of was 92.3% in the treatment-naïve group, 80% in the prior non-responders group, and 78.6% in the ineligible/intolerant group. Previous treatment history was a predictive factor for SVR12 in this prior and the present studies. Presently, the SVR12 rate of 89.7% in the treatment-naïve group was higher than the rate in the treatment failure group (81.8%), similar to a prior clinical study [10].
The presence of RAV was most important predictive factor of SVR in DCV+ASV treatment. Cases featuring positive RAV in a pretreatment examination are not eligible for insurance coverage in Korea. The prevalence of RAV is differs according to race and country; Japan has highest prevalence of RAV and non-Asian countries have lower prevalence of RAV than Asian countries [11-13]. The HALLMARK DUAL study, which included 78 Korean patients and 85 Taiwanese patients, reported an 11.8% (18/153) prevalence of RAV [9]. Among the 18 patients, 15 were Y93 positive and the remaining three were L31 positive. In the present study, 152 patients were examined for RAV; the resulting prevalence of 10.6% was similar with the prior study.
Presently, SVR12 in cases of positive RAV was markedly decreased. Similarly, a previous clinical study noted decreases from 95.9% to 58.8% in treatment-naïve patients, from 91.5% to 28% in previous non-responders, and from 90.1% to 36.7% in ineligible/intolerant patients [14]. This study also revealed similar results, although there were fewer RAV positive patients. RAV examinations in Korea are done at the Seoul Medical Science Institute using direct sequencing. If minor sequences of RAV are detected in more than 10% of the major sequence, the assigned result is RAV positive [15]. If the RAV including L31 and Y93 is lower than 10%, the result is negative. Whether a prevalence of RAV <10% influences SVR is still unclear.
Another factor affecting SVR12 is pretreatment HCV RNA level. The HALLMARK DUAL study reported an odds ratio of SVR12 of 3.38 for HCV RNA <800,000 IU/mL. SVR12 was higher with lower HCV RNA in treatment-naïve (96% vs. 87%) patients, previous non-responders (93% vs. 80%), and in ineligible/intolerant (88% vs. 80%) patients [14]. However, presently patients with HCV RNA ≥6 log10 IU/mL displayed higher SVR12 rate (90.5% vs. 88.2%). The present results should be interpreted cautiously, given the low numbers of patients in the groups.
DAA therapy including DCV+ASV is convenient and less often has adverse events compared to treatment involving peginterferon. The latter treatment can be stopped due to adverse events including myalgia, anemia, neutropenia, and depression, and has a reportedly low cure rate, especially in patients with cirrhosis [16,17]. In contrast, DCV+ASV produce mild adverse events in most clinical studies including patients with cirrhosis. Furthermore, SVR12 has been shown to be unaffected by presence of cirrhosis [14,18,19]. Presently, the SVR12 rate was lower in patients with cirrhosis. The dichotomy between prior and the present studies need clarification with more patients.
The most common adverse event of DCV+ASV is liver enzyme elevation; its prevalence is about 2% [14]. In this study, the prevalence of liver enzyme elevation was 2.6%. The American Association for the Study of Liver Disease and KASL guidelines recommend that drug treatment should be stopped when enzyme elevation is ≥10 times [2,3]. There was no discontinued case in our study. Skin problems can developed in all DAA treatment regimens, with a prevalence of up to 9.8% in combination with ribavirin and 3.8% without ribavirin [20]. Presently, four patients complained of skin problems and two stopped DCV+ASV treatment. It is very rare to stop DAA treatment due to skin problems. The patient who stopped the drug due to severe skin rash and itching achieved SVR12 even though medication was taken for only 4 months. This is similar with the patients who stopped the drug due to markedly elevated liver enzyme in previous clinical studies. This means there is a possibility to achieve SVR12 as long as medication is taken, even if the 24-week treatment regimen is not completed.
Up to 150 million individuals worldwide suffer from chronic HCV infection, with a prevalence of approximately 1% in Korea [8,21]. Therefore, the development of DAA is good news to patients with chronic hepatitis C. We confirmed that DCV+ASV in Korean patients has similar treatment efficacy and adverse events reported in prior clinical studies. But close monitoring to virologic breakthrough and severe adverse events are still needed.
Notes
Conflicts of Interest: The authors have no conflicts to disclose.
Abbreviations
ASV
asunaprevir
DAAs
direct acting antivirals
DCV
daclatasvir
ETR
end of treatment response
HCV
hepatitis C virus
LLOQ
lower limit of quantification
NS5A
non-structural 5A
RAV
resistance associated variant
SVR12
sustained virologic response at 12 weeks