The assessment of hepatocellular carcinoma risk in patients with chronic hepatitis B under antiviral therapy
Article information
Abstract
Hepatocellular carcinoma (HCC) is a primary concern for patients with chronic hepatitis B (CHB). Antiviral therapy has been reasonably the focus of interest for HCC prevention, with most studies reporting on the role of the chronologically preceding agents, interferon-alfa and lamivudine. The impact of interferon-alfa on the incidence of HCC is clearer in Asian patients and those with compensated cirrhosis, as several meta-analyses have consistently shown HCC risk reduction, compared to untreated patients. Nucleos(t)ide analogues also seem to have a favorable impact on the HCC incidence when data from randomized or matched controlled studies are considered. Given that the high-genetic barrier agents, entecavir and tenofovir, are mainly used in CHB because of their favorable effects on the overall long-term outcome of such patients, the most clinically important challenge is the identification of patients who require close HCC surveillance despite on-therapy virological remission. Several risk scores have been developed for HCC prediction in CHB patients. Most of them, such as GAG-HCC, CU-HCC and REACH-B, have been developed and validated in Asian untreated and treated CHB patients, but they do not seem to offer good predictability in Caucasian CHB patients for whom a newer score, PAGE-B, has been recently developed.
INTRODUCTION
Hepatocellular carcinoma (HCC) is currently the most serious complication of chronic hepatitis B virus (HBV) infection and one of the leading causes of cancer-related mortality worldwide [1]. Multiple HCC risk factors in chronic HBV patients have been described to date, including cirrhosis, older age, male sex, co-existence of alcohol abuse, diabetes or metabolic syndrome, active smoking, positive family history and others [2]. Additionally, HCC has been associated with some features pertaining to HBV infection, such as chronic necro-inflammatory activity, high HBV DNA and/or HBsAg levels, HBV genotype C (versus B) and presence of certain mutations, especially mutations in the basal core promoter region [3] or nonsense mutations in the surface gene (preS1 and preS2 regions).
HBV has high oncogenic potential itself and HBV-related carcinogenesis follows a multifactorial and multi-route process, which involves insertional mutagenesis following HBV DNA integration into host genome, increased genomic instability caused by HBV DNA integration and the direct effect of viral proteins, as well as dysregulation of normal cell functions (i.e. proliferation, apoptosis, DNA repair). This oncogenic activity is further enhanced in case of chronic active inflammation, during which increased oxidative stress and necrosis lead to subsequent regeneration, angiogenesis and cellular senescence, thus promoting mutagenesis and carcinogenesis. HBV DNA integration into the host genome has been shown to occur early in the phase of chronic HBV infection and at early steps of liver carcinogenesis [4]. The early HBV DNA integration may explain why non-cirrhotic chronic hepatitis B (CHB) patients under antiviral treatment can still carry a non-negligible risk of HCC development, which has considerable implications for their long-term monitoring. However, it is well recognized that the majority of HBV infected patients who are diagnosed with HCC have already developed cirrhosis.
The current management of CHB is based on therapy with interferon-alfa (IFNa) or a nucleos(t)ide analogue (NA). As long-standing high viral replication and active necro-inflammation have been associated with increased risk for HCC [5] in CHB patients, antiviral therapy which inhibits HBV replication and improves the necro-inflammatory activity is expected to decrease the HCC incidence. However, several studies suggest that HCC may still develop in treated CHB patients and it is debatable whether the HCC risk is decreased in CHB patients under antiviral therapy, particularly with the current treatment options, pegylated IFNa (peg-IFNa) or one of the high genetic barrier NAs, entecavir and tenofovir. In this review we sought to assess relevant evidence which evaluated the risk of HCC in CHB patients under treatment and determined predictors of HCC in this setting.
HCC RISK IN UNTREATED CHRONIC HBV PATIENTS
In a recent systematic review from Raffetti et al [2] which included 66 studies with 347,859 untreated patients, the summary HCC incidence rates ranged from 0.03 to 0.017 cases per 100 person-years (PYs) in inactive chronic HBV carriers, 0.12 to 0.49 cases per 100 PYs in CHB patients and 2.03 to 3.37 cases per 100 PYs in patients with HBV compensated cirrhosis. Accordingly, the 5-year cumulative HCC risks ranged from 0.1% to 0.3% in inactive carriers, 0.6% to 2.4% in CHB patients and 9.7% to 15.5% in cirrhotics, with the rates being higher in patients from East Asia rather than Europe. Multivariate analysis confirmed previous knowledge showing a significant increase of the HCC risk with more advanced phases of liver disease, older age, male gender, HBV genotype C and increasing levels of HBV DNA and HBsAg.
HCC RISK IN CHB PATIENTS TREATED WITH IFNa
Although peg-IFNa is practically the only IFNa currently used in the treatment of CHB, almost all studies assessing the HCC in IFNa treated CHB patients have used standard IFNa. Theoretically, IFNa therapy may decrease the HCC risk not only due to its antiviral but also due to its immunomodulatory and antitumoral properties. In agreement with the theoretical background, most published studies have shown a significant reduction of the HCC incidence risk in IFNa treated patients compared to untreated controls (Table 1). The first meta-analysis published in 2001 by Camma et al included 7 studies (2 Oriental, 5 European) with 1,505 cirrhotic patients and suggested that IFNa therapy can achieve 6.4% risk reduction in the incidence of HCC (P<0.001) [6]. The reduction of the HCC incidence resulted mainly from the two Oriental studies, while the 4.8% reduction of HCC incidence in the five European studies did not reach statistical significance. In another meta-analysis published in 2008 by Sung et al [7] including 12 studies with 2,742 patients, IFNa was found to reduce the HCC incidence by 34% over a follow-up of 4.9-8.9 years. More specifically, HCC developed in 4.6% in patients treated with IFNa and 9.0% in untreated controls (relative risk [RR]: 0.66, 95% confidence interval [CI]: 0.48-0.89). IFNa therapy offered no benefit in the low HCC incidence rates in non-cirrhotic patients (0.9% vs. 1.1%, RR: 0.72, 95% CI: 0.16–3.15), but a clear reduction in the HCC incidence in patients with cirrhosis (11.6% vs. 21.5%; RR: 0.53, 955 CI: 0.36-0.78). Similar findings were reported in four subsequent meta-analyses published in 2009-2011 [8-11].

Meta-analyses on hepatitis B virus related hepatocellular carcinoma incidence in patients treated with interferon-a
According to the existing data, IFNa and probably peg-IFNa seem to decrease the incidence of HCC, especially in Asian patients with cirrhosis who have high baseline risk for HCC. The HCC risk is lower in IFNa treated patients with sustained off-treatment virological response than non-sustained responders [7], but such a response can be achieved in up to 35% of HBeAg-positive and up to 25% of HBeAg-negative CHB patients who receive peg-IFNa [12].
Studies comparing the effect of IFNa and NAs on the prevention of HCC are generally lacking. In a very recent report by Lianq et al [13], however, treatment with peg-IFNa was associated with a lower HCC incidence compared to NA (P=0.011) or entecavir therapy (P=0.018) in 330 CHB patients, even after baseline matching, despite the more potent suppression of HBV replication by NAs.
HCC RISK IN CHB PATIENTS TREATED WITH NAs
Treated patients versus untreated controls
NAs represent the first-line treatment option for the majority of CHB patients because of the relatively low rates of sustained response, the possible contraindications, the poor tolerance and the patients’ unwillingness to receive peg-IFNa. Of the NAs, entecavir and tenofovir are mostly used due to their high potency and favorable resistance profile. However, given that lamivudine was the first chronologically available agent, most of the studies evaluating the effect of NAs on the HCC incidence have used lamivudine therapy.
In a landmark, randomized placebo-controlled trial by Liaw et al [14], 651 CHB patients (98% Asians, 85% males) with cirrhosis or advanced fibrosis were randomized to receive lamivudine (n=436) or placebo (n=215). Although the study was discontinued early after a median of 32 months due to significant improvement in the primary end points in the lamivudine group, a significant benefit in the HCC incidence was observed (lamivudine: 3.9% vs. placebo: 7.4%, P=0.047). When HCC cases diagnosed during the first year were excluded, the risk reduction became marginally non-significant (P=0.052), but a type II error related to the early trial termination could not be excluded.
Subsequent meta-analyses have confirmed the above findings. In one of them published by Papatheodoridis et al in 2010 [15], 21 studies with 3,881 CHB patients (33% cirrhotics, 49% HBeAg positive) treated with lamivudine and/or adefovir for a mean/median duration of ≥24 months were included. In the studies with both treated and untreated patients, the pooled HCC rate was higher in untreated (34/534 or 6.4%) than treated patients (22/779 or 2.8%, P=0.003) regardless of maintenance of on-therapy virological remission (Fig. 1). The overall pooled HCC incidence rates were higher in patients with than without cirrhosis (10.8% vs. 0.5%, P<0.001), with virological non-response or breakthroughs than maintained virological remission (7.5% vs. 2.3%, P<0.001) as well as in studies including patients with a mean/median age ≥50 than <50 years (6% vs. 2.8%, P<0.001) and in studies with predominantly (>85%) HBeAg negative than predominantly HBeAg positive patients (5.5% vs. 0.5%, P<0.001).
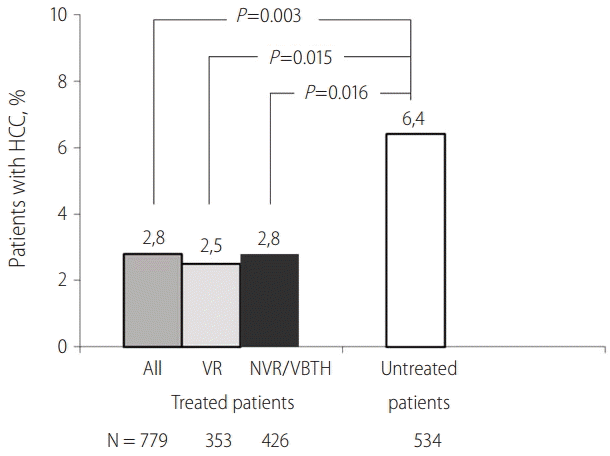
Incidence of hepatocellular carcinoma (HCC) in chronic hepatitis B patients treated with nucleos(t)ide analogues. Data from studies with treated patients and untreated controls included in a systematic review15. VR, virological remission; NVR, No virological response; VBTH:, virological breakthrough.
There is no randomized placebo-controlled trial in CHB patients with advanced liver disease in the era of the high genetic barrier NAs, entecavir and tenofovir. Given the great heterogeneity of the reported HCC incidence rates in several cohort studies including entecavir or tenofovir treated CHB patients [16], the safest approach for reaching conclusions on the effects of these NAs on the HCC risk is to focus on carefully designed matched controlled studies. The first such a study was published by Hosaka et al in 2013 [17] and compared three well matched historical cohorts of CHB patients (with or without cirrhosis): one was treated with entecavir, one was treated with lamivudine without any rescue therapy upon lamivudine resistance and one received no treatment. The cumulative HCC incidence rates did not differ among the three cohorts in the non-cirrhotic patients ranging from 2.5% to 3.6%, but it was significantly lower in the entecavir treated (7%) than in the lamivudine treated (22%, P=0,043 vs. entecavir) than in the untreated cohort (39%, P<0.001 vs. entecavir and P=0.019 vs. lamivudine) of cirrhotic patients.
In another large, retrospective-prospective study including 1,446 entecavir treated patients and 424 untreated controls [18], entecavir was found to reduce the 5-year incidence rates of HCC (13.8% vs. 26.4%, hazard ratio [HR]: 0.55; 95% CI: 0.31-0.99, P =0.049), hepatic events (HR: 0.51, 95% CI: 0.34-0.78, P=0.002), liver-related mortality (HR: 0.26; 95% CI: 0.13-0.55, P<0.001), and all-cause mortality (HR: 0.34; 95% CI: 0.18-0.62, P<0.001) in the cirrhotic patients but not in the total patient population. Similarly, in another cohort study by Yasunaka et al [19] including 1,206 CHB patients, entecavir significantly reduced the cumulative 5-year HCC incidence compared to lamivudine or no treatment (8.4% vs. 21.8% or 26.4%, P=0.013) in patients with age >35 years, HBV DNA > 4 log copies/mL and HBeAg seropositivity at diagnosis. In another study comparing CHB patients treated with NAs (n=117) and untreated controls matched by a propensity score (n=117), treatment was associated with significantly lower incidence of HCC (HR: 0.28, 95% CI: 0.13-0.62, P=0.002), with 5-year HCC incidence rates being 2.7% in patients on NAs and 11.3% in untreated patients [20].
Thus, the existing data support that NAs therapy reduces the risk of HCC in treated CHB patients, particularly cirrhotics, compared to matched untreated controls, but the HCC risk seems to remain higher in patients with maintained on-therapy virological remission compared to inactive chronic HBV carriers. This concept is supported by the findings of a large study by Cho et al [21], which compared the HCC incidence in 1378 CHB patients who were treated with NAs and 1014 inactive chronic HBV patients, including inactive cirrhotics, who remained untreated. The incidence of HCC was significantly higher among treated CHB patients despite of long-term virological remission or the presence of baseline cirrhosis (P<0.001), while independent risk factors for HCC were older age, male sex, baseline cirrhosis and initial group (active CHB vs. spontaneously inactive chronic HBV infection).
HCC risk in patients treated with different NAs
The majority of relevant studies has compared lamivudine with entecavir and has shown that maintenance of virological remission and not the agent itself is critical for the HCC risk under NAs therapy. In the previously mentioned study by Hosaka et al [17], entecavir was found to result in significantly lower 5-year cumulative HCC rates in cirrhotic patients, compared to lamivudine therapy (7% vs. 22%, P=0.043), but no rescue therapy upon lamivudine resistance was used. In a multicenter Greek study by Papatheodoridis et al [22], the HCC incidence was reported to be lower in 321 HBeAg-negative CHB patients treated with entecavir than in 818 patients treated initially with lamivudine, but the effect of the initial agent was not maintained in the multivariate analysis with adjustment for important HCC risk factors like age, gender and cirrhosis. In all other studies comparing the HCC incidence between CHB patients treated with entecavir or lamivudine with some rescue therapy upon lamivudine resistance, no difference in the HCC incidence between the two treatment approaches has been reported [23-25]. In the largest such study by Lim et al [23], the HCC rate was 2.41 cases per 100 PYs in entecavir treated patients (n=2,000) and 2.46 cases per 100 PYs in patients starting treatment with lamivudine (n=3,374) (HR: 1.01, 95% CI: 0.80-1.27).
Similarly, no difference on the HCC incidence has been found between entecavir and telbivudine [26,27] or between entecavir and tenofovir treated patients [28,29].
HCC RISK FACTORS IN PATIENTS TREATED WITH NAs
Older age, male gender and presence of cirrhosis represent widely accepted risk factors for HCC development in chronic HBV patients, which have been confirmed not only in untreated but in NAs treated patients as well [2,15,19,21].
In particular for NAs treated CHB patients, the role of on-therapy virological remission has been assessed in several studies. In the systematic review by Papatheodoridis et al [15] including mostly the early lamivudine studies with or without adefovir rescue therapy, failure to remain in virological remission under lamivudine was found to be associated with a higher HCC incidence particularly in cirrhotic patients. More recent studies further supported the concept that maintained on-lamivudine virological remission reduces the HCC incidence compared to patients with suboptimal responses or virological breakthroughs [30,31]. However, in a large Greek multicenter study [32] including 818 HBeAg-negative CHB patients who started therapy with lamivudine and received adefovir upon lamivudine resistance, the virological on-lamivudine remission was not found to significantly affect the HCC incidence. Similarly to the lamivudine data, virological remission on-entecavir has also been found to reduce the HCC incidence in recent studies [33,34]. A critical factor that may be responsible for the above discrepancies might be the proportion of patients with lamivudine resistance who received rescue therapy as well as differences in the follow-up of patients under lamivudine therapy and/or in the intervals from lamivudine resistance to rescue therapy.
Given that HBsAg loss is considered a difficult to be achieved but the optimal treatment end-point, the risk of HCC in patients who clear HBsAg under NAs is of particular interest. Recently, Kim et al [35] evaluated the HCC incidence (among other clinical outcomes) in 110 of 5,409 CHB patients who achieved HBsAg seroclearance over a median 6-year therapy with NAs. During 287 PYs following HBsAg seroclearance, only two patients (both with cirrhosis at baseline) developed HCC or died (0.7% annual risk) having a significantly lower rate compared to propensity-matched CHB patients without HBsAg seroclearance (HR 0.09, P<0.01). However, this study also confirmed that a residual HCC risk still exists even after HBsAg seroclearance.
The prognostic role of alpha fetoprotein (AFP) for HCC development has also been assessed in three studies including NAs treated patients. In a study by Wong et al [36] with 57 cases of HCC in 1,531 patients treated with entecavir for a mean of 51 months, baseline serum levels of AFP were predictive of HCC development. In this study, a baseline AFP cut-off value of 20 μg/L offered sensitivity 39% and specificity 99% for subsequent HCC development, while a lower cut-off of 6 μg/L increased the sensitivity to 81% but decreased the specificity of 80%. A study by Shim et al found a cumulative HCC incidence of 9.5% during 3 years of follow-up in 207 patients treated with NAs [37]. Serum AFP levels of 20 ng/dL at 12 months of therapy were strongly associated with HCC development offering positive predictive value of 100%. Finally, in the study by Yanq et al including 244 entecavir treated patients, HCC development was associated with persistently elevated AFP levels for at least 6 months of therapy [38].
THE ROLE OF HCC RISK SCORES
Individual HCC risk factors have been identified, but they cannot adequately classify CHB patients according to their HCC risk. Thus, there have been recent efforts for the development of accurate risk scores combining some of these risk factors that can predict the HCC risk in this setting.
HCC risk scores have been initially developed in untreated cohorts of Asian CHB patients. The most well-known such scores are CU-HCC, CAG-HCC and REACH-B (Table 2) [39-42]. The predictability of these scores has also been assessed in Asian CHB patients treated with NAs, mainly entecavir. In particular, Wonq et al. investigated the accuracy of these HCC risk scores in a cohort of 1531 CHB patients (22% with cirrhosis), treated with entecavir and followed for a mean of 42 months [43]. The 5-year cumulative HCC incidence rates were 12.9% in cirrhotics and 2.1% in non-cirrhotics, while the areas under the ROC curves of baseline CU-HCC, GAG-HCC, and REACH-B scores for HCC prediction were 0.80, 0.76, and 0.71, respectively. Moreover, CHB patients were reported to be at no or negligible HCC risk if they had low scores at both baseline and two years of therapy, at intermediate HCC risk if they had high score at baseline and low score at two years of therapy and at high HCC risk if they had high scores at both time points.
In three other studies comparing observed HCC incidence rates with that predicted by REACH-B score, the HCC incidence rates under NAs [44], entecavir [45] or tenofovir [46] were reported to be significantly lower than the expected HCC incidence rates according to the REACH-B score (standardized incidence ratios 0.37-0.46).
Unfortunately, the above HCC risk scores, which have been developed in Asian patients, do not seem to offer acceptable predictability for HCC in Caucasian CHB patients. In a large study by Papatheodoridis [29] including 1666 Caucasian CHB patients treated with entecavir and/or tenofovir, GAG-HCC, CU-HCC and REACH-B were associated with HCC development only in the univariate but not in the multivariate analyses and offered poor to modest predictability for HCC with areas under the ROC curve of 0.63-0.75. Similarly, in another multicenter study from 11 European referral centers including 744 entecavir treated CHB patients (42% Caucasian, 29% Asian, 19% other, 10% unknown), the HCC predictability of CU-HCC, GAG-HCC and REACH-B scores was low offering areas under the ROC curve of 0.54-0.74 [47].
Finally, a HCC risk score for Caucasian CHB patients under antiviral therapy was developed and validated in a recent study by Papatheodoridis et al [42]. They included 1815 adult Caucasian patients treated with entecavir or tenofovir for at least 12 months and developed a simple and accurate risk score for HCC development within 5 years of therapy, called PAGE-B, which includes platelets, age and gender (Table 2).
CONCLUSIONS
The risk of HCC merits special attention in the management of CHB due to its relatively high incidence even in patients who achieve long-standing viral suppression under treatment. Well known HBV-related HCC risk factors include older age, male sex, presence of cirrhosis and long-standing HBV replication, among others. The current armamentarium of antiviral drugs used in the treatment of CHB has improved the overall outcome of CHB patients but HCC may still develop. The effect of anti-HBV treatment in HCC prevention in CHB is more evident in patients with higher baseline HCC risk such as those with cirrhosis. Peg-IFNa probably decreases the HCC risk particularly in patients who achieve sustained off-treatment responses. NAs, which are used by the majority of CHB patients, achieve long-standing inhibition of HBV replication and seem to decrease but not eliminate the HCC risk, according to data from matched controlled studies. Thus, prediction of HCC risk remains of particular importance in patients under current therapies. In this effort, several HCC risk scores have been developed and offer good predictability for HCC mostly in Asian CHB patients. Recently, the PAGE-B risk score developed and seem to offer satisfactory performance in Caucasians CHB patients under the current oral agents.
Notes
Conflicts of Interest: Ioannis Varbobitis has no conflict to disclose. George V. Papatheodoridis has served as advisor/consultant/lecturer for Bristol-Myers Squibb, Gilead, Merck Sharp & Dohme, Novartis and Roche, has received research grants from Bristol-Myers Squibb, Gilead and Roche and has served in Data Safety Management Board for Gilead
Abbreviations
AFP
alpha fetoprotein
CHB
chronic hepatitis B
CI
confidence interval
HBV
hepatitis B virus
HCC
hepatocellular carcinoma
HR
hazard ratio
IFNa
interferon-alfa
NA
nucleos(t)ide analogue
peg-IFNa
pegylated IFNa
RR
relative risk
PYs
person-years
