Liver metastasis of meningeal hemangiopericytoma: a study of 5 cases
Article information
Abstract
Mesenchymal tumors in the liver, whether primary or metastatic, are rare. Meningeal hemangiopericytoma (HPC) is characteristically associated with delayed metastasis and the liver is one of the most common sites. Despite its consistent histological features, a pathological diagnosis of HPC in the liver is sometimes not straightforward due to its rarity and usually remote medical history of the primary meningeal tumor. In this report, the clinicopathological features of 5 cases of metastatic HPC to the liver were reviewed and described.
INTRODUCTION
Solitary fibrous tumor (SFT), previously known as hemangiopericytoma (HPC), is a mesenchymal tumor primarily involving the soft tissue and head and neck region. SFT classically comprises spindle cells of uniform cellularity and moderate pleomorphism, together with the presence of staghorn vascular channels. Immunohistochemically, HPC cells stain positively with bcl-2 and CD99, and in some cases CD34. Meningeal HPC shares common features with SFT of the soft tissue in terms of genetic and immunohistochemical characteristics. NAB2-STAT6 gene fusion and STAT6 immunopositivity were consistently detected in both entities [1-4]. Besides, meningeal HPC is a histological mimicker of meningioma and other spindle cell tumors such as solitary fibrous tumor. A distinction of meningeal HPC from the differential diagnoses is important and carries clinical implications since HPC is an aggressive tumor associated with recurrence and distant metastasis. The 5-year and 15-year local recurrence rate was noted to be 46% and 92% respectively [5]. The extracranial metastatic rate ranged from 12.9% to 26% in three large cohorts each comprising more than 30 cases [5-7]. Distant metastasis of HPC most of the time signifies a poor prognosis. The liver is a common site of metastasis. In this report we described five cases of liver metastasis from meningeal hemangiopericytoma and reviewed the clinicopathological features.
MATERIALS AND METHODS
Five cases of HPC metastasis to the liver were retrieved from the archives at the Department of Pathology, Queen Mary Hospital, Hong Kong, Department of Pathology, Dartmouth-Hitchcock Medical Center, New Hampshire, USA, and Robert J. Tomsich Pathology & Laboratory Medicine Institute and Department of Cancer Biology, Cleveland Clinic and Lerner Research Institute, USA. Clinical and pathological features were reviewed and analyzed. Clinical and follow-up data were obtained from the clinical patient records.
RESULTS
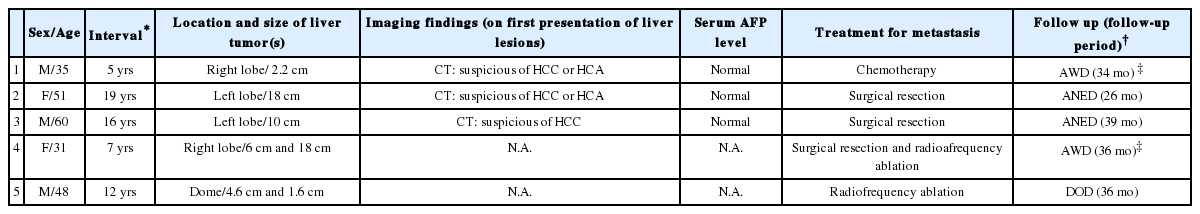
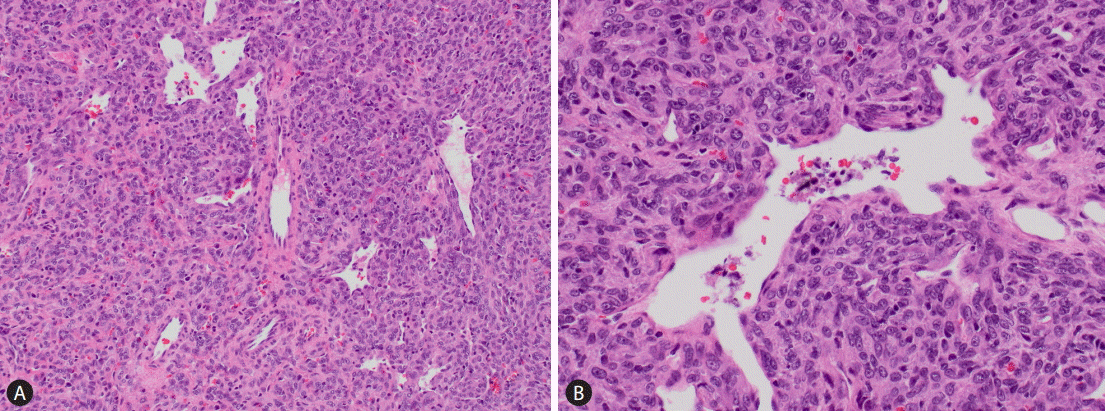
The clinicopathological data of the five cases were summarized in Table 1. The patients ranged from 31 years to 60 years. The interval between the diagnoses of the primary meningeal tumor to that of liver metastasis ranged from 5 years to 19 years. The tumor size ranged from 1.6 cm to 18 cm. All patients were not known to be carriers for hepatitis B or hepatitis C virus; and none of them was cirrhotic. The serum alpha-fetoprotein (AFP) level was within the normal range in all the 3 patients from whom data were available. Radiological findings suggested the possibility of hepatocytic lesions such as hepatocellular carcinoma (HCC) or hepatocellular adenoma. Three patients were treated with surgical resection; one of them received concomitant radiofrequency ablation. The fourth patient received radiofrequency ablation only; while the last received systemic chemotherapy for disseminated disease. Histologically, the metastatic tumors in the liver showed characteristic features of HPC with sheets of spindle cells of uniform hypercellularity and the presence of staghorn blood vessels. The tumor cells were homogeneous with abundant cytoplasm, oval nuclei, and moderate pleomorphism (Fig. 1). The follow-up period ranged from 26 to 39 months. Two patients were lost follow-up at the time of writing. Among the remaining three patients, one died of disease at 36 months; the other two were alive with no evidence of disease at 26 and 39 months respectively.
DISCUSSION
While majority (59%) of meningeal HPC recurs in the central nervous system at the primary site [8], extracranial metastasis is also encountered. Metastasis of HPC developed at an average of 107 months and 123 months respectively in two studies [5,7]. In other words, metastasis is usually delayed and may occur years or even decades after diagnosis of the primary tumors. Extracranial metastastic sites can be varied and include the pancreas, bone and the liver [9-11], etc., in which the bone and the liver are the most common sites (82% and 41% respectively) [8].
Hepatic mesenchymal tumors, primary or metastatic, are not common when compared with hepatocytic tumors or metastasis from extra-hepatic carcinomas or melanomas. Clinically, some patients present with symptoms of hypoglycemia, which is associated with production of insulin-like growth factor II by the tumor [12]. The sizes of the liver metastatic HPC tumors in our report varied from 1.6 cm to 1.8 cm and presented as solitary as well as multiple tumor nodules. In the case of solitary lesions, they may clinically mimic a primary liver tumor such as hepatocellular adenoma or hepatocellular carcinoma.
It is known that meningeal HPC is associated with delayed metastasis and in our current report, the longest interval to liver metastasis was 19 years. Pre-operative diagnosis may be difficult, especially when medical history (of a mengingeal HPC) is incomplete or unavailable. Radiologically, in the primary site of the central nervous system, HPC can be differentiated from meningioma with its osteolytic effect and absence of dural tail sign [13,14]. When it comes to metastatic sites such as the liver, imaging may not be a reliable means to distinguish metastatic hepatic HPC from HCC as the enhancement characteristics are similar to other hypervascular tumors such as HCC [15]. The low update of FDG on Fluorodeoxyglucose (FDG) PET may be able to suggest a diagnosis of metastatic HPC [16].
Microscopic examination is crucial for diagnosis. Monomorphous spindle cells of moderate cellularity and round to oval nuclei are typically seen, arranged around staghorn blood vessels. The histological differential diagnoses include other types of mesenchymal tumors such as synovial sarcoma, leiomyosarcoma, or (metastatic) gastrointestinal stromal tumor. A careful examination of the histological features and ancillary immunohistochemical stains including the recently discovered STAT6 are essential to arrive at the correct diagnosis.
Surgical resection remains the mainstay of treatment for hepatic metastatic HPC. Radiotherapy provides tumor control and symptomatic relief [8]. Liver transplantation has also been reported as a treatment option [17,18]. Anti-angiogenic agents are potential promising therapeutic options for treatment of HPC [19,20].
In conclusion, liver metastasis of meningeal hemangiopericytoma carries a typical protracted clinical course. It is a rare event among the various neoplasms occurring in the liver. Identification of salient histological features, a high index of suspicion and correlation with clinical history are helpful to achieve a correct diagnosis.
Notes
Conflicts of Interest: The authors have no conflicts to disclose.
Abbreviations
AFP
alpha-fetoprotein
FDG
Fluorodeoxyglucose
HCC
hepatocellula