| Korean J Hepatol > Volume 18(1); 2012 > Article |
ABSTRACT
Background/Aims
Hepatocellular carcinoma (HCC), which is the third most common cancer in Korea, has a very poor prognosis. However, only a few studies have performed a comprehensive survival-related analysis in all patients who were consecutively diagnosed and treated over a given period of time. The aim of this study was to determine the 5-year survival rate and its prognostic factors among HCC patients.
Methods
In total, 257 patients who were consecutively diagnosed with HCC between January 2000 and December 2003 were followed until death or until December 2008. We analyzed their survival outcomes according to their clinical characteristics, tumor staging, and treatment modalities, and determined the independent prognostic factors affecting survival.
Results
The patients were aged 59┬▒10 years (mean┬▒SD). During the follow-up period, 223 patients (86.8%) died and the overall median survival was 10.8 months; the 1-, 3-, and 5-year survival rates were 44.4%, 21.0%, and 12.1%, respectively. The outcomes in patients with tumor node metastasis (TNM) stage I or II and Child-Pugh class A or B were significantly better with surgical resection than with other treatment modalities (P<0.01). Patients who underwent supplementary transcatheter arterial chemoembolization as a second-line treatment after surgical resection had better outcomes than those who underwent surgical resection alone (P=0.02). Initial symptoms, Child-Pugh class, serum alpha-fetoprotein, tumor size, portal vein thrombosis, and TNM stage were found to be independent prognostic factors for survival among HCC patients.
Hepatocellular carcinoma (HCC), which shows a grave morbidity and mortality, is the third most common malignancy following stomach and lung cancers in Korea.1,2 HCC has a very poor prognosis in comparison with other solid tumors because HCC rapidly progresses with its aggressive biological behavior and is usually diagnosed at an advanced stage. Furthermore, the majority of HCC develops in the background of chronic liver diseases such as liver cirrhosis, which makes a curative treatment more difficult.3 Nevertheless, the 5-year survival rate of HCC has slightly improved from 11.0% in 1993-1997 to 14.7% in 1998-2002 in Korea,4 and several large-scale studies have also reported modest improvements in the survival of HCC patients worldwide during the last few decades.5,6 It is expected that the survival rates of HCC patients will continue to increase with strict application of screening/surveillance programs using alpha-fetoprotein (AFP) and abdominal ultrasonography and with exquisite development of treatment modalities, such as liver transplantation, hepatic resection, radiofrequency ablation (RFA), transcatheter arterial chemoembolization (TACE), and molecularly targeted therapies. Although many studies reported outcomes of HCC patients according to the treatment modalities,7-13 only a few studies have reported survivalrelated analysis in all patients who were consecutively diagnosed and treated in a single institution in Korea.14
The aim of this study was to perform survival analysis and to identify possible prognostic factors for survival of HCC patients who were diagnosed and treated for a given period of time, thereby providing useful information on predicting prognostic factors according to clinical characteristics, tumor staging, and treatment modalities.
Between January 2000 and December 2003, 308 patients who were diagnosed as HCC at Dankook University Hospital were retrospectively enrolled. HCC was diagnosed according to the guidelines of the Korea Liver Cancer Study Group and the National Cancer Center.14 Fifty-one patients with inadequate data, prior initial treatments for HCC at other hospitals, or interruption to follow up were excluded, leaving 257 patients followed until death or until December 2008.
Patients received one of five initial treatment modalities: hepatic resection, RFA, TACE, systemic chemotherapy, or supportive care. The choice of initial treatment was based on the Child-Pugh classification and tumor staging (Fig. 1). RFA was performed percutaneously using Cool tip electrodes (Valleylab, Burlington, MA, USA) under ultrasound guidance. TACE was performed using adriamycin-lipiodol emulsion and/or absorbable gelatin sponge particles (Gelfoam, Upjohn Co., MI, USA). Computed tomography (CT) or magnetic resonance imaging (MRI) and the serum AFP level were checked every three to six months after curative treatments and every two to three months after TACE. If an evidence of tumor recurrence was noted in these tests, TACE was performed as a second-line treatment.
We reviewed patients' records and examined the following demographic and clinical parameters: age, sex, initial symptoms, tumor etiology, biochemistry with liver tests, and hematological and radiological data. All data including Child-Pugh classification and modified 5th UICC/TNM tumor stage15 were determined at the time of HCC diagnosis. We analyzed the survival of patients according to initial treatments: surgical resection, RFA, TACE, systemic chemotherapy, or supportive care; we also subanalyzed in two parts of the HCC patients who received TACE as a second-line treatment: TACE after surgical resection and TACE after RFA. The study was performed in compliance with the ethics principles of the Declaration of Helsinki with Good Clinical Practice guidelines.
Statistical analysis was performed with SPSS version 12.0 software (SPSS Inc., Chicago, IL, USA). Survival curves were obtained and verified by the Kaplan-Meier method with a log-rank test. To identify baseline predictors of survival at the time of HCC diagnosis, univariate and multivariate regression analysis were performed using the log-rank test and Cox's proportional hazard regression model, respectively. Statistical significance was defined as a
P-value less than 0.05.
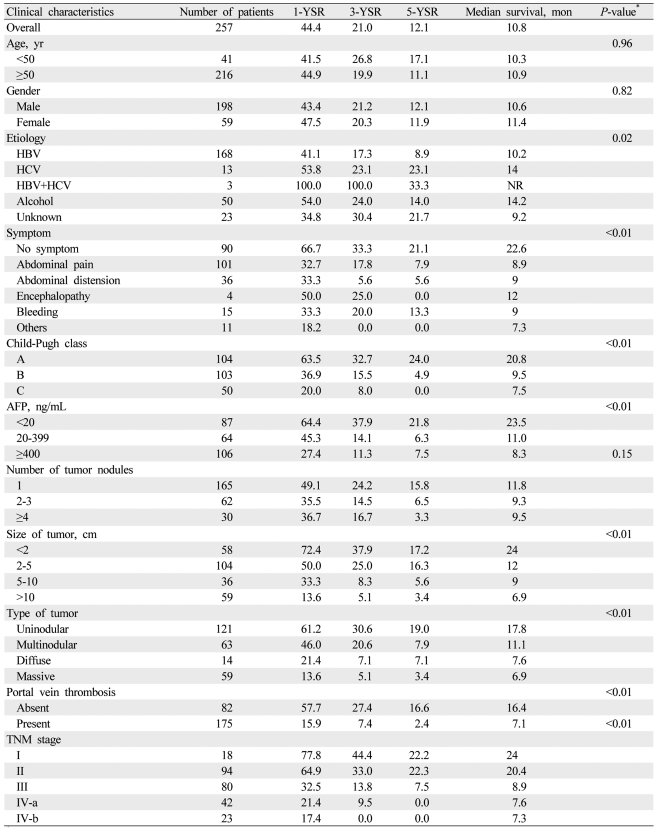
Baseline characteristics are summarized in Table 1. Male gender (77%) was predominant, and the mean age was 59 ┬▒ 10 years. Hepatitis B virus (65.4%) was the most common underlying cause of HCC, and abdominal pain (39.3%) was the most common initial presenting symptom. Two hundred seven patients (80.5%) were Child-Pugh class A or B. One hundred sixty-five patients (64.2%) had a single nodule, and 175 patients (68.1%) had accompanying portal vein thrombosis. Serum AFP levels were elevated above 400 ng/mL in 106 patients (41.2%). One hundred forty-five patients (56.4%) were classified as TNM stage III or IV.
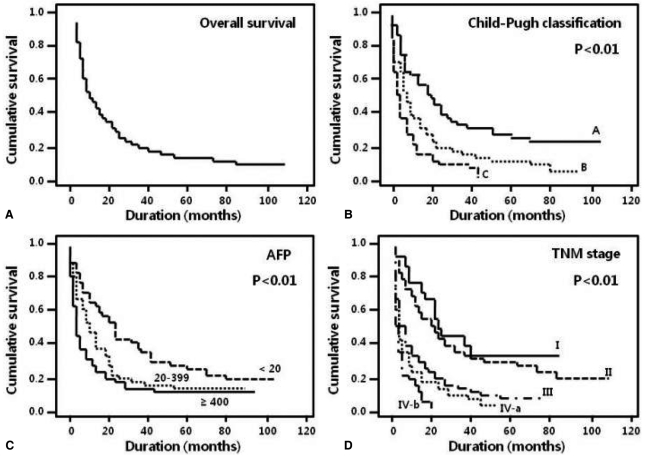
Two hundred twenty-three patients (86.8%) died during the follow-up period, and 1-, 3-, and 5-year survival rates were 44.4%, 21.0% and 12.1%, respectively. The overall median survival of all patients was 10.8 months (Table 1, Fig. 2A). The 5-year survival rate of patients without symptoms was higher than that of patients with at least one symptom (21.1% vs. 13.3% [bleeding], P<0.01; Table 1). The 5-year survival rate of patients with Child-Pugh class A was higher than that of patients with class B (24.0% vs. 4.9%, P<0.01; Table 1). None of the patients with Child-Pugh class C survived 5 years; their median survival was 7.5 months (Fig. 2B). The 5-year survival rate of patients with an AFP level less than 20 ng/mL was higher than that of patients with an AFP level equal to or more than 400 ng/mL (21.8% vs. 7.5%, P<0.01; Table 1, Fig. 2C). The 1-, 3-, and 5-year survival rates were significantly different according to tumor characteristics, such as size, type, and portal vein thrombosis (Table 1). The TNM staging system showed a significant difference in the probability of survival across the different stages (P<0.01). Patients with TNM stage I and II had a median survival of 24 and 20.4 months, respectively, and patients with TNM stage IV had a median survival of less than 8 months (Table 1, Fig. 2D).
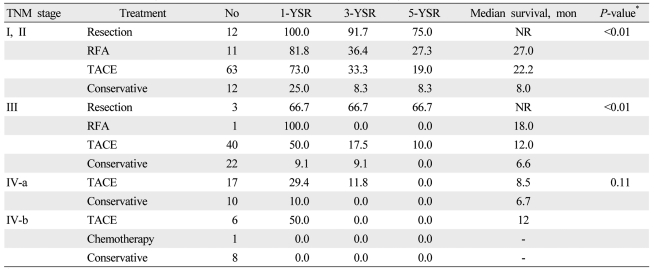
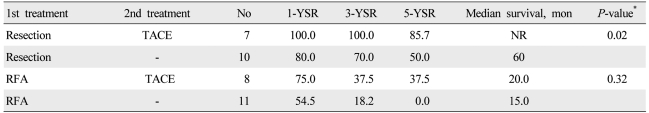
For initial treatment, 17 patients (6.6%) underwent surgical resection, 19 (7.4%) underwent RFA, 135 (52.5%) underwent TACE, 2 (0.8%) received systemic chemotherapy, and 84 (32.7%) received supportive care. The survival of the patients was analyzed on the basis of the initial treatment adopted in patients with Child-Pugh class A or B (Table 2). In patients with TNM stage I and II, the 5-year survival rates of patients who underwent surgical resection, RFA, and TACE were significantly different (75.0%, 27.3%, and 19.0%; P<0.01). In patients with TNM stage III, the 5-year survival rate of patients who underwent surgical resection was higher than that of patients who received TACE (66.7% vs. 10.0%; P<0.01). No significant difference was observed in survival rates between the patients with TNM stage IV-a and IV-b. In analysis of the patients who received supplementary TACE as a second-line treatment (Table 3), the 5-year survival rate of patients who underwent TACE after surgical resection was higher than that of patients who underwent only surgical resection (85.7% vs. 50%; P=0.02). No significant difference was seen in survival rates between the patients who underwent TACE after RFA and those who underwent only RFA.
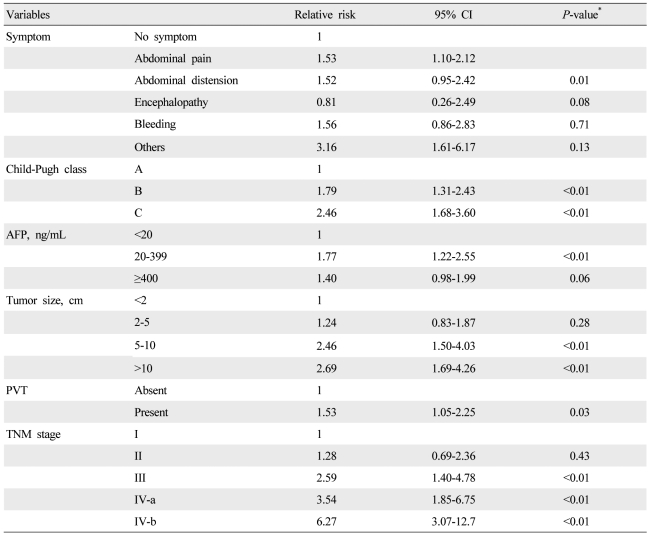
Univariate analysis showed that etiology (P=0.02), initial symptom (P<0.01), Child-Pugh class (P<0.01), AFP level (P<0.01), tumor size (P<0.01), tumor type (P<0.01), portal vein thrombosis (P<0.01), and TNM staging (P<0.01) were significant baseline predictors of survival in patients with HCC (Table 1). Multivariate analysis identified initial symptom, Child-Pugh class, AFP level, tumor size, portal vein thrombosis, and TNM stage as independent baseline predictors of survival (Table 4).
Despite the high prevalence and mortality of HCC in Korea, few studies have stratified the survival outcomes according to liver functions, tumor stages, and treatment modalities in a cohort of HCC patients.14 Most studies released limited reports about survival outcomes in HCC patients who underwent a certain therapy.16,17 In this study, clinical data were collected from all patients who were treated and followed at a single center for a given period of time and analyzed according to the clinical staging system and treatment modalities proposed by the Korea Liver Cancer Study Group and the National Cancer Center.18 We expect that this study can provide appropriate information about the clinical characteristics, prognostic factors, and survival outcomes of HCC patients in Korea.
In the present study, the overall 5-year survival rate of HCC patients was not higher than that reported through two population-based studies in the years 1998 to 2002,3,4 which might be caused by the inability of patients with relatively early stages and preserved liver function to receive curative treatment. About 12% of patients with Child-Pugh class A or B and TNM stage I or II received only conservative treatment because of poor economic status and inadequate cooperation to treatment.
When planning an optimal treatment for HCC patients, considering hepatic reserve function as well as tumor stage is very important. Although the TNM staging system for HCC does not consider variables indicative of liver function, this system is still sufficiently powerful enough to predict a patient's prognosis and to aid in determining treatment options with the assistance of laboratory parameters readily available in clinical settings. Without exception even in this study, this staging system, as expected, revealed a significant difference in the probability of survival across the different stages in HCC patients.
Besides tumor stage and remaining liver function, treatment modality is one of the most important factors directly affecting the survival and prognosis of patients.19 In patients with relatively preserved liver function, such as those in Child-Pugh class A or B and early TNM stage I or II, the median survival period of patients who underwent surgical resection could not be estimated due to not reaching a survival rate of 50%, while the median survival period of patients who received RFA or TACE were 27 or 22.2 months, respectively, which was longer than that reported previously.20-22 Additionally, in patients with TNM stage III, surgical resection statistically showed the best treatment results; however, it is inappropriate to grant its clinical significance because the number of patients who received this operation in this stage was limited, as most patients received TACE.
Park et al23 reported that the one-, three-, and five-year survival rates after TACE were 61%, 29%, and 15%, similar to the results of our study. TACE can improve the survival of patients with unresectable HCC;24,25 in the present study, the survival rates of patients who underwent TACE were higher than those who received conservative treatment. Interestingly, the median survival of patients treated with TACE was shorter in TNM stage IV-a than in those with TNM stage IV-b (8.5 vs. 12 months). This result may reflect that the portal vein invasion has a worse influence than distant metastasis on survival for HCC patients with preserved liver function.
As HCC is frequently observed by recurrence or metastasis after curative management, such as surgical resection or RFA,26-29 multimodality treatment has been frequently performed. This study analyzed survival rates in patients who received TACE as a second-line treatment modality after surgical resection or RFA. When compared with resection only, second-line TACE after resection revealed significantly better results in survival rates and median survival periods. Second-line TACE after RFA also showed longer survival times even if it was not statistically significant when compared with RFA only. TACE is a treatment modality most frequently applied to patients with HCC, and its survival benefit is known.30,31 The beneficial role of TACE as a second-line therapy in HCC could be expected. However, TACE in the present study was applied to the patients with an evidence or suspicion of recurrence after curative treatments such as resection or RFA in terms of optional therapy but not adjuvant one. Further study including a large number of patients undergoing multimodality treatments should be performed through a prospective cohort of HCC.
Several investigations analyzed predictive factors for survival of patients with HCC.14,32,33 In our study, initial symptoms, Child-Pugh classification, serum AFP level, tumor size, portal vein thrombosis, and TNM staging were identified as independent prognostic factors for HCC. However, this is a single-center study, and liver transplantation was not available in our institution during this study period, which is possibly a limiting factor with respect to generalizing these results.
In summary, we identified the clinical characteristics, survival rates, and independent prognostic factors affecting survival in a retrospective cohort of HCC patients. This cohort analysis may provide useful information of prognosis predictability in HCC patients, especially for a prospective cohort study in the future.
Acknowledgements
All authors who have contributed to the present study declared that they had nothing to disclose any conflict of interest and any financial support in connection with this manuscript.
REFERENCES
1. Song IH, Kim KS. Current status of liver disease in Korea: hepatocellular carcinoma. Korean J Hepatol 2009;15(Suppl 6):S50-S59. 20037280.


2. Korea National Statistical Office. Annual report on the cause of death statistics 2005. 2006. Daejeon: Korea National Statistical Office; p. 6-15.
3. Park JW. National policy of early detection in hepatocellular carcinoma. Korean J Hepatol 2002;8(Suppl 3):S16-S16.
4. Jung KW, Yim SH, Kong HJ, Hwang SY, Won YJ, Lee JK, et al. Cancer survival in Korea 1993-2002: a population-based study. J Korean Med Sci 2007;22(Suppl):S5-S10. 17923755.



5. Capocaccia R, Sant M, Berrino F, Simonetti A, Santi V, Trevisani F. EUROCARE Working Group. Hepatocellular carcinoma: trends of incidence and survival in Europe and the United States at the end of the 20th century. Am J Gastroenterol 2007;102:1661-1670. 17555459.


6. Toyoda H, Kumada T, Kiriyama S, Sone Y, Tanikawa M, Hisanaga Y, et al. Changes in the characteristics and survival rate of hepatocellular carcinoma from 1976 to 2000: analysis of 1365 patients in a single institution in Japan. Cancer 2004;100:2415-2421. 15160346.


7. Lee JG, Kang CM, Park JS, Kim KS, Yoon DS, Choi JS, et al. The actual five-year survival rate of hepatocellular carcinoma patients after curative resection. Yonsei Med J 2006;47:105-112. 16502491.



8. Park YK, Kim BW, Wang HJ, Kim MW. Hepatic resection for hepatocellular carcinoma meeting milan criteria in Child-Turcotte-Pugh class a patients with cirrhosis. Transplant Proc 2009;41:1691-1697. 19545709.


9. Seo HI, Park SJ, Kim SH, Lee WJ, Ahn M, Park HS, et al. Prognostic factor analysis of 200 consecutive hepatic resections for hepatocellular carcinoma. Korean J Hepatobiliary Pancreat Surg 2006;10:21-28.
10. Sung YM, Choi D, Lim HK, Lee WJ, Kim SH, Kim MJ, et al. Long-term results of percutaneous ethanol injection for the treatment of hepatocellular carcinoma in Korea. Korean J Radiol 2006;7:187-192. 16969048.



11. Cho CM, Tak WY, Kweon YO, Kim SK, Choi YH, Hwang YJ, et al. The comparative results of radiofrequency ablation versus surgical resection for the treatment of hepatocellular carcinoma. Korean J Hepatol 2005;11:59-71. 15788886.

12. Lee JH, Han SY, Jo JH, Kim SK, Go BS, Oh JY, et al. Prognostic factors for survival in patients with hepatocellular carcinoma after radiofrequency ablation. Korean J Gastroenterol 2007;49:17-23. 18167429.

13. Seong J, Lee IJ, Shim SJ, Lim do H, Kim TH, Kim JH, et al. A multicenter retrospective cohort study of practice patterns and clinical outcome on radiotherapy for hepatocellular carcinoma in Korea. Liver Int 2009;29:147-152. 18795897.


14. Park KW, Park JW, Kim TH, Choi JI, Kim SH, Park HS, et al. Five-year survival analysis of a cohort of hepatocellular carcinoma patients who treated at the National Cancer Center, Korea. Korean J Hepatol 2007;13:530-542. 18159151.


15. Sobin LH, Fleming ID. Union Internationale Contre le Cancer and the American Joint Committee on Cancer. TNM Classification of Malignant Tumors, fifth edition (1997). Cancer 1997;80:1803-1804. 9351551.


16. Han DH, Choi GH, Kim DH, Choi SB, Kang CM, Kim KS, et al. Single center experience (ten years) with surgical resection for treating hepatocellular carcinoma: strategies for improving the long-term survival after resection. Korean J Hepatobiliary Pancreat Surg 2008;12:245-253.
17. Lee JK, Chung YH, Song BC, Shin JW, Choi WB, Yang SH, et al. Recurrences of hepatocellular carcinoma following initial remission by transcatheter arterial chemoembolization. J Gastroenterol Hepatol 2002;17:52-58. 11895553.


18. Park JW. Korean Liver Cancer Study Group and National Cancer
Center. Practice guideline for diagnosis and treatment of hepatocellular carcinoma. Korean J Hepatol 2004;10:88-98. 15218342.

19. Park KW, Park JW, Choi JI, Kim TH, Kim SH, Park HS, et al. Survival analysis of 904 patients with hepatocellular carcinoma in a hepatitis B virus-endemic area. J Gastroenterol Hepatol 2008;23:467-473. 17764529.


20. Kanematsu T, Furui J, Yanaga K, Okudaira S, Shimada M, Shirabe K. A 16-year experience in performing hepatic resection in 303 patients with hepatocellular carcinoma: 1985-2000. Surgery 2002;131(1 Suppl):S153-S158. 11821803.


21. Lee CS, Sheu JC, Wang M, Hsu HC. Long-term outcome after surgery for asymptomatic small hepatocellular carcinoma. Br J Surg 1996;83:330-333. 8665183.


22. Llovet JM. Updated treatment approach to hepatocellular carcinoma. J Gastroenterol 2005;40:225-235. 15830281.


23. Park JH, Chung JW, Lee SK, Han JK, Lee HS, Kim CY, et al. Chemoembolization of hepatocellular carcinoma: long-term survival and prognostic factors. J Korean Radiol Soc 1996;35:315-323.

24. Takayasu K, Arii S, Ikai I, Omata M, Okita K, Ichida T, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology 2006;131:461-469. 16890600.


25. Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology 2003;37:429-442. 12540794.


26. Choi GH, Han DH, Kim DH, Choi SB, Kang CM, Kim KS, et al. Outcome after curative resection for a huge (>or =10 cm) hepatocellular carcinoma and prognostic significance of gross tumor classification. Am J Surg 2009;198:693-701. 19268907.


27. Wang HJ, Lee H. Surgical treatment of hepatocellular carcinoma. Korean J Gastroenterol 2005;45:247-257. 15843750.

28. Cho HW, Lee JG, Lim CY, Kang CM, Kim KS, Choi JS, et al. Factors affecting the recurrence of hepatocellular carcinoma after surgical resection. J Korean Surg Soc 2005;69:465-470.
29. Chung IK, Park MJ, Kwon KT, Park YD, Chung YJ, Jeon SW, et al. The factors related to the prognosis of solitary hepatocellular carcinoma after radiofrequency ablation. Korean J Hepatol 2005;11:371-380. 16380666.

30. Lee HS, Kim JS, Choi IJ, Chung JW, Park JH, Kim CY. The safety and efficacy of transcatheter arterial chemoembolization in the treatment of patients with hepatocellular carcinoma and main portal vein obstruction. A prospective controlled study. Cancer 1997;79:2087-2094. 9179054.


31. Kim KM, Kim JH, Park IS, Ko GY, Yoon HK, Sung KB, et al. Reappraisal of repeated transarterial chemoembolization in the treatment of hepatocellular carcinoma with portal vein invasion. J Gastroenterol Hepatol 2009;24:806-814. 19207681.


Figure┬Ā1
Treatment algorithm for hepatocellular carcinoma (HCC). RFA, radiofrequency ablation; TACE, transcatheter arterial chemoembolization.
Figure┬Ā2
Kaplan-Meier survival curves according to clinical and tumor characteristics. (A) The overall median survival of the 257 patients was 10.8 months. (B) The 5-year survival rates of patients with Child-Pugh class A, B, and C were 24, 4.9, and 0%, respectively (P<0.01). (C) The 5-year survival rate was higher in patients with an alpha-fetoprotein (AFP) level of <20 ng/mL than in those with an AFP level of Ōēź20 ng/mL (P<0.01). (D) Analysis of tumor node metastasis (TNM) staging revealed that the probability of survival differed significantly with the stage (P<0.01).
Table┬Ā1
Hepatocellular carcinoma (HCC) survival rates according to clinical and tumor characteristics
Table┬Ā2
Survival rates according to treatment modalities and tumor node metastasis (TNM) stage in patients with Child-Pugh class A or B



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




