| Korean J Hepatol > Volume 17(3); 2011 > Article |
ABSTRACT
Primary biliary cirrhosis (PBC) is a slowly progressive cholestatic liver disease of autoimmune etiology. The initial presentation of PBC is various from asymptomatic, abnormal liver biochemical tests to overt cirrhosis. The diagnosis of PBC is based on cholestatic biochemical liver tests, presence of antimitochondrial antibody and histologic findings of nonsuppurative destructive cholangitis. Although the diagnosis is straightforward, it could be underdiagnosed because of its asymptomatic presentation, or underrecognition of the disease. UDCA in a dose of 13-15 mg/kg is the widely approved therapy which can improve the prognosis of patients with PBC. However, one-third of patients does not respond to UDCA therapy and may require liver transplantation. Every effort to diagnose PBC in earlier stage and to develop new therapeutic drugs and clinical trials should be made.
Primary biliary cirrhosis (PBC) is a slowly progressive cholestatic liver disease of autoimmune etiology.1 PBC is characterized by presence of antimichondrial antibody (AMA), histologic findings of portal inflammation and immune-mediated destruction of the intrahepatic bile ducts. It mainly affects middle-aged women. PBC is most prevalent in northern Europe. The prevalence of PBC differs considerably in different geographic regions, ranging from 40 to 400 per million.1 The prevalence of PBC in Japan is about from 27 to 54 per million.2 The prevalence of PBC in Korea has not been investigated, but PBC is designated as one of rare disorders by Korean government. The clinical characteristics of PBC in Korea are similar with those in regions where PBC are prevalent.3 The manifestations and prognosis are various in different patients. Diagnosis in earlier stage and treatment with ursodeoxycholic acid (UDCA) have improved the prognosis in patients with PBC over the past two decades. This article reviews an overview of the updated knowledge on the diagnosis and treatment of PBC.
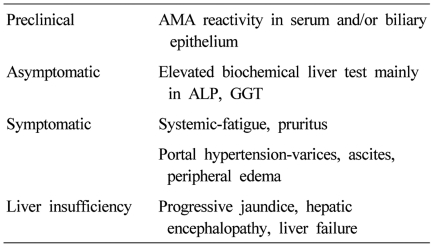
PBC progresses insidiously through the clinical phases: preclinical, asymptomatic, symptomatic, and liver insufficiency (Table 1).4 The preclinical phase is characterized by AMA reactivity with no symptom and normal biochemical liver tests. Then patients develop biochemical abnormalities but remain asymptomatic. The median time to progression from preclinical to asymptomatic phase was 5.6 years (range, 1-20 years).5 Asymptomatic phase is followed by the development of symptoms, usually fatigue and pruritus, and later varices, edema, or ascites in most untreated patients within 2 to 4 years.6 Liver insufficiency is characterized by accelerated jaundice, and the prognosis is poor.7 Mean survival in patients with bilirubin level of 2.0 mg/dL is 4 years, and that in patients with bilirubin level of 6.0 mg/dL is 2 years.
The prognosis of patients with PBC has improved significantly over the past 2 decades because more patients are being diagnosed earlier in the disease process8 and being treated with UDCA. UDCA therapy significantly delayed histologic progression,9 decreased the development of esophageal varices,10 and increased the survival in patients with PBC.11-13 The survival rate of patients with early stage (stage 1 or 2 disease) who were treated with UDCA for a mean of eight years was similar to that of a healthy control population.14
PBC is now diagnosed earlier in its clinical course owing to easy access to biochemical tests and widespread use of the specific AMA assay. More than 50% of patients are asymptomatic at presentation.3,15-17 Sixty percents of patients were asymptomatic at diagnosis also in Korea.3 The most common symptoms in PBC patients at diagnosis are fatigue and pruritus. Fatigue has been reported in up to 78 percents of patients,18-20 and does not appear to correlate with disease severity, histologic stage, or duration, and may impair the quality of life.20 The etiology of fatigue is unknown, but may be related to autonomic dysfunction.21 Pruritus, which occurs in 20 to 70 percent of patients, can be the most distressing symptom.22 The onset of pruritus usually precedes the onset of jaundice by months to years. The pruritus can be local or diffuse. It is usually worse at night and is often exacerbated by contact with wool, other fabrics, or heat. Its cause is unknown, but endogenous opioids may have a role. Unexplained discomfort in the right upper quadrant occurs in approximately 10 percent of patients.23 Other common findings in primary biliary cirrhosis include hyperlipidemia, hypothyroidism, osteopenia, and coexisting autoimmune diseases such as Sjögren's syndrome and scleroderma.24 Portal hypertension does not usually occur until later in the course of the disease. Malabsorption, deficiencies of fat-soluble vitamins, and steatorrhea are uncommon except in advanced disease. Rarely, patients present with ascites, hepatic encephalopathy, or hemorrhage from esophageal varices.25 The incidence of hepatocellular carcinoma is elevated among patients with long-standing advanced disease.26 Other diseases associated with primary biliary cirrhosis include interstitial pneumonitis, celiac disease, sarcoidosis, renal tubular acidosis, hemolytic anemia, and autoimmune thrombocytopenia.
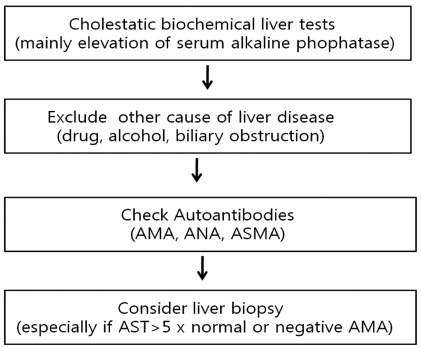
The diagnosis of PBC should be suspected in the setting of chronic cholestasis after exclusion of other causes. The diagnosis is based on the following findings; 1) biochemical evidence of cholestasis with elevated alkaline phophatase (ALP) activity and/or gamma glutamyl transpeptidase (GGT), 2) presence of antimitochondiral antibody (AMA), and 3) histologic evidence of nonsuppurative cholangitis and destruction of interlobular bile ducts (Fig. 1). Patients are diagnosed as probable PBC if two of these three features are present after exclusion of biliary obstruction.27
The biochemical hallmarks of PBC are elevated serum ALP and GGT. Mild elevation of serum aminotransferases and increased level of immunoglobulins (mainly IgM) is commonly observed16 while some patients with PBC may have high aminotransferase activities with hypergammaglobulinemia. The changes in biochemical tests reflect in part the severity of histology28,29 and the improvement of biochemical tests after UDCA administration is a strong predictor of long-term prognosis.30-33 In patients without cirrhosis, the degree of ALP elevation is related to the severity of ductopenia and inflammation on liver histology. The increase in aminotransferase and IgG levels reflects the degree of periportal and lobular necroinflammation. The level of serum bilirubin reflect the severity of ductopenia and biliary piecemeal necrosis.29 Hyperbilirubinemia, hypergammaglobulinemia, hypoalbuminemia, and thrombocytopenia are indicators of the development of liver cirrhosis and portal hypertension. As in other chronic cholestatic disease, serum cholesterol levels often elevated.28,29
AMA was described in a patient with PBC in 1965 for the first time,34 and has been regarded as a hallmark of PBC. Serum AMA is highly specific for the diagnosis of PBC and detected in nearly 95% of patients with PBC, while it is detected in normal population in about 1%.1 When AMA is detected in asymptomatic subjects with normal biochemical tests, PBC is already present histologically in 40% of cases,35 and in the remaining patients it is likely to develop in succeeding years.5,16,36,37 The antigenic target of AMA is the E2 subunits of 2-oxo acid dehydrogenase complexes, in particular the pyruvate dehydrogenase complex (PDC)-E2.38 AMA titer may differ by more than 200-fold among patients who have PBC, but in the single patient it remains stable over the years, and has no prognostic value in PBC.39 Therefore, presence of AMA itself rather than its titer is important for the diagnosis. The measurement of serum AMA is typically based on the immunofluorescent techniques (the criteria of positivity, above 1:40), however, with recognition of antigenic determinants, enzyme-linked immunosorbent assays (ELISA) or western blotting assays have been developed. Each assay uses different epitopes, so that interpretation of AMA result should be considered the different sensitivity and specificity of the specific assay. Moreover, 5-10% of PBC patients did not show AMA positivity in their sera, so that liver biopsy is required for the suspicious cases. However, the comparison of AMA-positive PBC and AMA-negative PBC did not show significant differences in terms of clinical features, treatment response or prognosis.40 Antinuclear antibodies (ANA) and anti-smooth muscle antibody (ASMA) are found in about half of PBC patients. ANAs such as anti-gp210 and possibly anti-p62 are detected in some of patients with PBC and may be associated with aggressive disease and poor prognosis.41-43
Histology of PBC is characterized by chronic, nonsuppurative cholangitis that affects interlobular and septal bile ducts. The term "florid duct lesion" is often used when focal lesions show intense inflammatory changes and necrosis around the small bile ducts. The inflammatory infiltrates are comprised of plasma cells, macrophages, polymorphonuclear cells (especially eosinophils) and sometimes epithelioid granulomas.1 The size of the specimen is important and at least 10-15 portal tracts should be present to adequately evaluate cholangitis and ductopenia.
Histologic lesions are classically divided into four stages. Stage I is characterized by portal inflammation with or without florid duct lesion and the inflammation is confined to the portal triads. Stage II is a progression of periportal lesions to involvement of the hepatic parenchyma, which is termed as interface hepatitis. Stage III is characterized by distortion of the hepatic architecture with numerous fibrous septa. Stage IV is defined as cirrhosis with the existence of regenerative nodules.44
The role of liver biopsy for the diagnosis of PBC is limited, when the biochemical liver tests and AMA results are compatible to PBC. However, the information on the stage of PBC and the exclusion of the possibility of overlap syndrome such as autoimmune hepatitis can be obtained from the liver biopsy results. For AMA-negative patients, liver biopsy is mandatory for the diagnosis of PBC. Therefore, balanced decision should be made to do or not to do liver biopsy for suspicious PBC patients after consideration of benefit and risk or cost related to the invasive procedure.
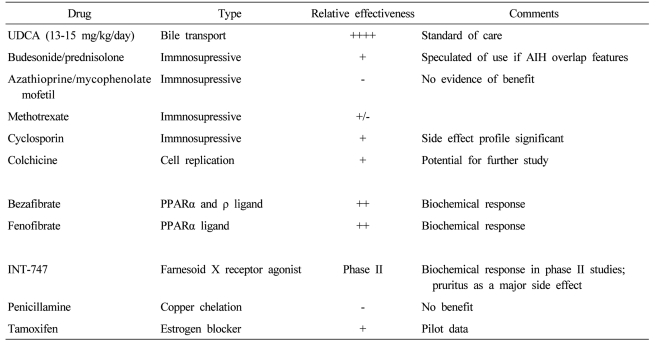
UDCA, the 7-beta epimer of chenodeoxycholic acid, comprised 2% of human bile acid and has several interrelated functions including direct choleretic, anti-inflammatory, and antiapoptotic properties.45 UDCA in a dose of 13-15 mg/kg /day is the only drug approved for PBC treatment by USA FDA (Table 2).27 It decreases serum levels of bilirubin, ALP, aminotransferase, cholesterol and IgM,46,47 and improves liver histology.12,46 A combined analysis of the three largest clinical trials shows that UDCA prolongs survival free of liver transplantation.48 The adequate dosing of UDCA is important. A dose of 13-15 mg/kg/day was superior to either a lower dose of 5-7 mg/kg/day or a higher dose of 23-25 mg/kg/day in biochemical responses and costs.49 A complete response occurs in about 30% of patients treated with PBC, which was defined by normalized biochemical tests and stabilized or improved liver histology.50,51 Serum ALP levels at 6 months after UDCA therapy can be helpful in predicting response to UDCA.30,32 The life expectancy of patients showing the complete response during treatment with UDCA was similar to that of age- and sex-matched healthy controls for up to 20 years.32 However, the disease progresses in many patients who do not show the complete response during UDCA therapy, for whom additional medical treatment is definitely required.
Other drugs for the treatment of PBC have been studied for the past decade as single agents or adjuvant medications. None of these drugs have been found beneficial as single agents, in which colchicines,52,53 methotraxate,54 penicillamine,55 cyclosporine,56 corticosteroid,57 azathioprine,58 mycophenolate mofetil were included.59 Many of these have been used in combination with UDCA to see if further improvement in liver disease can be achieved. Budesonide had been reported to improve liver histology and the biochemical tests of liver function when used with UDCA, but it may worsen osteopenia.60 However, studies were of too short treatment duration to show convincingly whether budesonide will improve survival or not. The additions of colchicines,61 methotraxate62 and silymarin63 to UDCA had no additional benefits compared with UDCA alone.
Bezafibrate and fenofibrate (fibric acid derivatives used to treat hypertriglyceridemia) improved liver biochemical tests in pilot studies.64,65 The proposed mechanism of action of fibric acid derivatives in treatment of PBC involves the regulation of expression of immunomodulatory proteins and lipids,66,67 downregulation of cholesterol 7 alpha-hydroxylase, an enzyme involved in the synthesis of bile acids68 and decrease of multi-drug resistance (MDR) gene through the activation of peroxisome proliferator-activated receptor alpha.69 Farnesoid X receptor (FXR) is a bile acid-activated nuclear receptor highly expressed in both the liver and gastrointestinal tract. It has a regulatory role in bile and cholesterol metabolism, and FXR agonists such as INT-747 may hold promise for the new therapeutic option in UDCA-refractory PBC.70 Modification of UDCA (nor-UDCA) with more potent choleretic property than UDCA improved liver biochemistry and histology in MDR2 knockout mouse, an animal model for slcerosing cholangitis, which suggests that translational studies in human are required.71 Tamoxifen decreased alkaline phosphatase levels in two women who were taking it after surgery for breast cancer.72 Although atorvastatin with many antiinflammatory properties was commonly used for control of hypercholesterolemia in PBC, it was not effective for PBC itself.73
Transplantation is the only effective treatment for those with decompensated cirrhosis or liver failure.74 The outcome of liver transplantation in patients with PBC is more favorable than for other liver diseases. The survival rates at one and five years are 92 percent and 85 percent, respectively. About 20-25% of patients with PBC who undergo transplantation have recurrent disease over 10 years. Fortunately, recurrent PBC does not affect patient or graft survival.75
The diagnosis of PBC should be suspected in subjects with chronic cholestasis, mainly elevation of ALP after exclusion of other causes of hepatobiliary disease. The diagnosis of PBC is largely confirmed with tests for AMA. AMA is highly specific for the diagnosis of PBC and positive in nearly 95% of patients. Liver biopsy can be used especially for further evaluation in subjects with negative tests for AMA and suspicious overlap syndrome. UDCA in a dose of 13-15 mg/kg /day is the standard therapy for PBC treatment, however, about 40% of the patients do not respond to UDCA. Therefore, further efforts to develop new drugs and clinical trial should be warranted.
REFERENCES
2. Inoue K, Hirohara J, Nakano T, Seki T, Sasaki H, Higuchi K, et al. Prediction of prognosis of primary biliary cirrhosis in Japan. Liver 1995;15:70-77. 7791541.


3. Kim KA, Jeong SH, Lee JI, Yeon JE, Lee HJ, Kwon SY, et al. Clinical features and prognosis of primary biliary cirrhosis in Korea. Korean J Hepatol 2010;16:139-146. 20606498.


4. Mayo MJ. Natural history of primary biliary cirrhosis. Clin Liver Dis 2008;12:277-288. viii. 18456180.


5. Metcalf JV, Mitchison HC, Palmer JM, Jones DE, Bassendine MF, James OF. Natural history of early primary biliary cirrhosis. Lancet 1996;348:1399-1402. 8937278.


6. Balasubramaniam K, Grambsch PM, Wiesner RH, Lindor KD, Dickson ER. Diminished survival in asymptomatic primary biliary cirrhosis. A prospective study. Gastroenterology 1990;98:1567-1571. 2338193.


7. Shapiro JM, Smith H, Schaffner F. Serum bilirubin: a prognostic factor in primary biliary cirrhosis. Gut 1979;20:137-140. 428825.



8. Prince MI, James OF. The epidemiology of primary biliary cirrhosis. Clin Liver Dis 2003;7:795-819. 14594132.


9. Poupon RE, Lindor KD, Parés A, Chazouillères O, Poupon R, Heathcote EJ. Combined analysis of the effect of treatment with ursodeoxycholic acid on histologic progression in primary biliary cirrhosis. J Hepatol 2003;39:12-16. 12821038.


10. Lindor KD, Jorgensen RA, Therneau TM, Malinchoc M, Dickson ER. Ursodeoxycholic acid delays the onset of esophageal varices in primary biliary cirrhosis. Mayo Clin Proc 1997;72:1137-1140. 9413293.


11. Lindor KD, Therneau TM, Jorgensen RA, Malinchoc M, Dickson ER. Effects of ursodeoxycholic acid on survival in patients with primary biliary cirrhosis. Gastroenterology 1996;110:1515-1518. 8613058.


12. Poupon RE, Balkau B, Eschwège E, Poupon R. UDCA-PBC Study Group. A multicenter, controlled trial of ursodiol for the treatment of primary biliary cirrhosis. N Engl J Med 1991;324:1548-1554. 1674105.


13. Poupon RE, Poupon R, Balkau B. The UDCA-PBC Study Group. Ursodiol for the long-term treatment of primary biliary cirrhosis. N Engl J Med 1994;330:1342-1347. 8152446.


14. Corpechot C, Carrat F, Bahr A, Chrétien Y, Poupon RE, Poupon R. The effect of ursodeoxycholic acid therapy on the natural course of primary biliary cirrhosis. Gastroenterology 2005;128:297-303. 15685541.


15. Parés A, Rodés J. Natural history of primary biliary cirrhosis. Clin Liver Dis 2003;7:779-794. 14594131.


16. Prince MI, Chetwynd A, Craig WL, Metcalf JV, James OF. Asymptomatic primary biliary cirrhosis: clinical features, prognosis, and symptom progression in a large population based cohort. Gut 2004;53:865-870. 15138215.



17. Kim WR, Lindor KD, Locke GR 3rd, Therneau TM, Homburger HA, Batts KP, et al. Epidemiology and natural history of primary biliary cirrhosis in a US community. Gastroenterology 2000;119:1631-1636. 11113084.


18. Goldblatt J, Taylor PJ, Lipman T, Prince MI, Baragiotta A, Bassendine MF, et al. The true impact of fatigue in primary biliary cirrhosis: a population study. Gastroenterology 2002;122:1235-1241. 11984509.


19. Forton DM, Patel N, Prince M, Oatridge A, Hamilton G, Goldblatt J, et al. Fatigue and primary biliary cirrhosis: association of globus pallidus magnetisation transfer ratio measurements with fatigue severity and blood manganese levels. Gut 2004;53:587-592. 15016756.



20. Poupon RE, Chrétien Y, Chazouillères O, Poupon R, Chwalow J. Quality of life in patients with primary biliary cirrhosis. Hepatology 2004;40:489-494. 15368455.


21. Newton JL, Gibson GJ, Tomlinson M, Wilton K, Jones D. Fatigue in primary biliary cirrhosis is associated with excessive daytime somnolence. Hepatology 2006;44:91-98. 16800007.


22. Talwalkar JA, Souto E, Jorgensen RA, Lindor KD. Natural history of pruritus in primary biliary cirrhosis. Clin Gastroenterol Hepatol 2003;1:297-302. 15017671.


23. Laurin JM, DeSotel CK, Jorgensen RA, Dickson ER, Lindor KD. The natural history of abdominal pain associated with primary biliary cirrhosis. Am J Gastroenterol 1994;89:1840-1843. 7942679.

24. Watt FE, James OF, Jones DE. Patterns of autoimmunity in primary biliary cirrhosis patients and their families: a population-based cohort study. QJM 2004;97:397-406. 15208427.


25. Nakanuma Y. Are esophagogastric varices a late manifestation in primary biliary cirrhosis? J Gastroenterol 2003;38:1110-1112. 14673735.


26. Nijhawan PK, Therneau TM, Dickson ER, Boynton J, Lindor KD. Incidence of cancer in primary biliary cirrhosis: the Mayo experience. Hepatology 1999;29:1396-1398. 10216121.


27. Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ. American Association for Study of Liver Diseases. Primary biliary cirrhosis. Hepatology 2009;50:291-308. 19554543.


28. Corpechot C, Poujol-Robert A, Wendum D, Galotte M, Chrétien Y, Poupon RE, et al. Biochemical markers of liver fibrosis and lymphocytic piecemeal necrosis in UDCA-treated patients with primary biliary cirrhosis. Liver Int 2004;24:187-193. 15189267.


29. Poupon R, Chazouillères O, Balkau B, Poupon RE. UDCA-PBC Group. Clinical and biochemical expression of the histopathological lesions of primary biliary cirrhosis. J Hepatol 1999;30:408-412. 10190722.


30. Angulo P, Lindor KD, Therneau TM, Jorgensen RA, Malinchoc M, Kamath PS, et al. Utilization of the Mayo risk score in patients with primary biliary cirrhosis receiving ursodeoxycholic acid. Liver 1999;19:115-121. 10220741.


31. Corpechot C, Abenavoli L, Rabahi N, Chretien Y, Andreani T, Johanet C, et al. Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology 2008;48:871-877. 18752324.


32. Parés A, Caballería L, Rodés J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic Acid. Gastroenterology 2006;130:715-720. 16530513.


33. Kuiper EM, Hansen BE, de Vries RA, den Ouden-Muller JW, van Ditzhuijsen TJ, Haagsma EB, et al. Improved prognosis of patients with primary biliary cirrhosis that have a biochemical response to ursodeoxycholic acid. Gastroenterology 2009;136:1281-1287. 19208346.


34. Walker JG, Doniach D, Roitt IM, Sherlock S. Serological tests in diagnosis of primary biliary cirrhosis. Lancet 1965;1:827-831. 14263538.


35. Mitchison HC, Bassendine MF, Hendrick A, Bennett MK, Bird G, Watson AJ, et al. Positive antimitochondrial antibody but normal alkaline phosphatase: is this primary biliary cirrhosis? Hepatology 1986;6:1279-1284. 3793004.


36. Springer J, Cauch-Dudek K, O'Rourke K, Wanless IR, Heathcote EJ. Asymptomatic primary biliary cirrhosis: a study of its natural history and prognosis. Am J Gastroenterol 1999;94:47-53. 9934730.


37. Prince M, Chetwynd A, Newman W, Metcalf JV, James OF. Survival and symptom progression in a geographically based cohort of patients with primary biliary cirrhosis: follow-up for up to 28 years. Gastroenterology 2002;123:1044-1051. 12360466.


38. Fussey SP, Guest JR, James OF, Bassendine MF, Yeaman SJ. Identification and analysis of the major M2 autoantigens in primary biliary cirrhosis. Proc Natl Acad Sci U S A 1988;85:8654-8658. 3186751.



39. Van Norstrand MD, Malinchoc M, Lindor KD, Therneau TM, Gershwin ME, Leung PS, et al. Quantitative measurement of autoantibodies to recombinant mitochondrial antigens in patients with primary biliary cirrhosis: relationship of levels of autoantibodies to disease progression. Hepatology 1997;25:6-11. 8985257.


40. Gisbert JP, Jones EA, Pajares JM, Moreno-Otero R. Review article: is there an optimal therapeutic regimen for antimitochondrial antibody-negative primary biliary cirrhosis (autoimmune cholangitis)? Aliment Pharmacol Ther 2003;17:17-27. 12492729.


41. Muratori P, Muratori L, Ferrari R, Cassani F, Bianchi G, Lenzi M, et al. Characterization and clinical impact of antinuclear antibodies in primary biliary cirrhosis. Am J Gastroenterol 2003;98:431-437. 12591064.


42. Invernizzi P, Podda M, Battezzati PM, Crosignani A, Zuin M, Hitchman E, et al. Autoantibodies against nuclear pore complexes are associated with more active and severe liver disease in primary biliary cirrhosis. J Hepatol 2001;34:366-372. 11322196.


43. Granito A, Muratori P, Muratori L, Pappas G, Cassani F, Worthington J, et al. Antibodies to SS-A/Ro-52kD and centromere in autoimmune liver disease: a clue to diagnosis and prognosis of primary biliary cirrhosis. Aliment Pharmacol Ther 2007;26:831-838. 17767467.


44. Kumagi T, Onji M. Presentation and diagnosis of primary biliary cirrhosis in the 21st century. Clin Liver Dis 2008;12:243-259. vii. 18456178.


45. Lazaridis KN, Gores GJ, Lindor KD. Ursodeoxycholic acid 'mechanisms of action and clinical use in hepatobiliary disorders'. J Hepatol 2001;35:134-146. 11495032.


46. Heathcote EJ, Cauch-Dudek K, Walker V, Bailey RJ, Blendis LM, Ghent CN, et al. The Canadian Multicenter Double-blind Randomized Controlled Trial of ursodeoxycholic acid in primary biliary cirrhosis. Hepatology 1994;19:1149-1156. 8175136.


47. Parés A, Caballería L, Rodés J, Bruguera M, Rodrigo L, García-Plaza A, et al. Long-term effects of ursodeoxycholic acid in primary biliary cirrhosis: results of a double-blind controlled multicentric trial. UDCA-Cooperative Group from the Spanish Association for the Study of the Liver. J Hepatol 2000;32:561-566. 10782903.


48. Poupon RE, Lindor KD, Cauch-Dudek K, Dickson ER, Poupon R, Heathcote EJ. Combined analysis of randomized controlled trials of ursodeoxycholic acid in primary biliary cirrhosis. Gastroenterology 1997;113:884-890. 9287980.


49. Angulo P, Dickson ER, Therneau TM, Jorgensen RA, Smith C, DeSotel CK, et al. Comparison of three doses of ursodeoxycholic acid in the treatment of primary biliary cirrhosis: a randomized trial. J Hepatol 1999;30:830-835. 10365809.


50. Jorgensen RA, Dickson ER, Hofmann AF, Rossi SS, Lindor KD. Characterisation of patients with a complete biochemical response to ursodeoxycholic acid. Gut 1995;36:935-938. 7615288.



51. Leuschner M, Dietrich CF, You T, Seidl C, Raedle J, Herrmann G, et al. Characterisation of patients with primary biliary cirrhosis responding to long term ursodeoxycholic acid treatment. Gut 2000;46:121-126. 10601067.



52. Kaplan MM, Alling DW, Zimmerman HJ, Wolfe HJ, Sepersky RA, Hirsch GS, et al. A prospective trial of colchicine for primary biliary cirrhosis. N Engl J Med 1986;315:1448-1454. 3537784.


53. Gong Y, Gluud C. Colchicine for primary biliary cirrhosis. Cochrane Database Syst Rev 2004;(2):CD004481. 15106254.


54. Hendrickse MT, Rigney E, Giaffer MH, Soomro I, Triger DR, Underwood JC, et al. Low-dose methotrexate is ineffective in primary biliary cirrhosis: long-term results of a placebo-controlled trial. Gastroenterology 1999;117:400-407. 10419922.


55. Dickson ER, Fleming TR, Wiesner RH, Baldus WP, Fleming CR, Ludwig J, et al. Trial of penicillamine in advanced primary biliary cirrhosis. N Engl J Med 1985;312:1011-1015. 3885033.


56. Lombard M, Portmann B, Neuberger J, Williams R, Tygstrup N, Ranek L, et al. Cyclosporin A treatment in primary biliary cirrhosis: results of a long-term placebo controlled trial. Gastroenterology 1993;104:519-526. 8425695.


57. Mitchison HC, Palmer JM, Bassendine MF, Watson AJ, Record CO, James OF. A controlled trial of prednisolone treatment in primary biliary cirrhosis. Three-year results. J Hepatol 1992;15:336-344. 1447500.


58. Christensen E, Neuberger J, Crowe J, Altman DG, Popper H, Portmann B, et al. Beneficial effect of azathioprine and prediction of prognosis in primary biliary cirrhosis. Final results of an international trial. Gastroenterology 1985;89:1084-1091. 3899841.


59. Talwalkar JA, Angulo P, Keach JC, Petz JL, Jorgensen RA, Lindor KD. Mycophenolate mofetil for the treatment of primary biliary cirrhosis in patients with an incomplete response to ursodeoxycholic acid. J Clin Gastroenterol 2005;39:168-171. 15681915.


60. Angulo P, Jorgensen RA, Keach JC, Dickson ER, Smith C, Lindor KD. Oral budesonide in the treatment of patients with primary biliary cirrhosis with a suboptimal response to ursodeoxycholic acid. Hepatology 2000;31:318-323. 10655252.


61. Battezzati PM, Zuin M, Crosignani A, Allocca M, Invernizzi P, Selmi C, et al. Ten-year combination treatment with colchicine and ursodeoxycholic acid for primary biliary cirrhosis: a double-blind, placebo-controlled trial on symptomatic patients. Aliment Pharmacol Ther 2001;15:1427-1434. 11552915.


62. Combes B, Emerson SS, Flye NL, Munoz SJ, Luketic VA, Mayo MJ, et al. Methotrexate (MTX) plus ursodeoxycholic acid (UDCA) in the treatment of primary biliary cirrhosis. Hepatology 2005;42:1184-1193. 16250039.


63. Angulo P, Patel T, Jorgensen RA, Therneau TM, Lindor KD. Silymarin in the treatment of patients with primary biliary cirrhosis with a suboptimal response to ursodeoxycholic acid. Hepatology 2000;32:897-900. 11050036.


64. Nakai S, Masaki T, Kurokohchi K, Deguchi A, Nishioka M. Combination therapy of bezafibrate and ursodeoxycholic acid in primary biliary cirrhosis: a preliminary study. Am J Gastroenterol 2000;95:326-327. 10638623.


65. Levy C, Peter JA, Nelson DR, Keach J, Petz J, Cabrera R, et al. Pilot study: fenofibrate for patients with primary biliary cirrhosis and an incomplete response to ursodeoxycholic acid. Aliment Pharmacol Ther 2011;33:235-242. 21083674.


66. Gebel T, Arand M, Oesch F. Induction of the peroxisome proliferator activated receptor by fenofibrate in rat liver. FEBS Lett 1992;309:37-40. 1324848.


67. Schoonjans K, Staels B, Auwerx J. Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J Lipid Res 1996;37:907-925. 8725145.


68. Roglans N, Vázquez-Carrera M, Alegret M, Novell F, Zambón D, Ros E, et al. Fibrates modify the expression of key factors involved in bile-acid synthesis and biliary-lipid secretion in gallstone patients. Eur J Clin Pharmacol 2004;59:855-861. 14685799.


69. Matsumoto T, Miyazaki H, Nakahashi Y, Hirohara J, Seki T, Inoue K, et al. Multidrug resistance3 is in situ detected in the liver of patients with primary biliary cirrhosis, and induced in human hepatoma cells by bezafibrate. Hepatol Res 2004;30:125-136. 15588777.


70. Lindor KD. Farnesoid X receptor agonists for primary biliary cirrhosis. Curr Opin Gastroenterol 2011;27:285-288. 21297469.


71. Fickert P, Wagner M, Marschall HU, Fuchsbichler A, Zollner G, Tsybrovskyy O, et al. 24-norUrsodeoxycholic acid is superior to ursodeoxycholic acid in the treatment of sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology 2006;130:465-481. 16472600.


72. Reddy A, Prince M, James OF, Jain S, Bassendine MF. Tamoxifen: a novel treatment for primary biliary cirrhosis? Liver Int 2004;24:194-197. 15189268.


73. Stojakovic T, Putz-Bankuti C, Fauler G, Scharnagl H, Wagner M, Stadlbauer V, et al. Atorvastatin in patients with primary biliary cirrhosis and incomplete biochemical response to ursodeoxycholic acid. Hepatology 2007;46:776-784. 17668874.


- TOOLS
-
METRICS

- Related articles
-
The current trends in the health burden of primary liver cancer across the globe2023 April;29(2)
Clinical and serological prognostic markers in primary biliary cholangitis2021 April;27(2)
Changes in the epidemiology and management of bacterial infections in cirrhosis2021 July;27(3)
Current understanding of primary biliary cholangitis2021 January;27(1)
Radiomics and radiogenomics of primary liver cancers2019 March;25(1)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



