Cholangiocarcinoma arising in von Meyenburg complexes
Article information
INTRODUCTION
Von Meyenburg complexes (VMCs) are a rare incidental premortem finding. They are developmental defects, rather than a true neoplasm, that are found relatively frequently at laparotomy and necropsy. Although VMCs were originally reported to occur in only 0.6-0.7% of surgical and autopsy specimens,1,2 a recent study of consecutive autopsies found that the incidence was approximately 5.6% in adults and 0.9% in children.3 The question of malignant transformation of hepatic VMCs is controversial.4 Malignant transformation of VMCs to intrahepatic cholangiocarcinoma (CC) is very rare, with only 35 cases (including 5 Korean cases) described in the English literature to date.5-8 Herein we report a case of intrahepatic CC arising from VMCs in an elderly man and a brief review of the pathologic findings is presented.
CASE SUMMARY
A 66-year-old male was transferred to our hospital for the evaluation and treatment of a hepatic mass that was found during the preoperative workup for knee replacement surgery. This patient had been diagnosed with hypertension 20 years previously. The results of a physical examination were unremarkable, and those of liver function tests were normal with the exception of a high serum gamma-glutamyl transpeptidase level (307 IU/L). Serum alpha-fetoprotein and carcinoembryonic antigen levels were 6.5 ng/mL and 1.54 ng/mL, respectively, and he was negative for both hepatitis B surface antigen and antibody to hepatitis C virus. An abdominal computed tomography scan revealed two lobulated masses with a bulging contour on the right lobe that were distinct from each other. Given a suspicion of hepatocellular carcinoma, the patient submitted to a right liver lobectomy. At 10 months after the operation he was well, with no evidence of a tumor.
PATHOLOGIC FINDINGS
The resected portion of the right liver had dimensions of 16×14×7 cm and weighed 480 g. Centrally there was an ill-demarcated subcapsular mass intermingled with cystic and solid areas, measuring 7.0×6.5×5.0 cm; a surgical margin of at least 1.0 cm around the mass had been ensured. The cut surface of the solid part was pinkish-white and granular, without hemorrhage or necrosis. The cystic portion had spongiotic cut surfaces and ranged from 0.1 to 0.3 cm in diameter (Fig. 1). Another lobulated subcapsular mass, measuring 1.5×1.5×1.0 cm, was circumscribed, yellow-tan, and firm. The remaining hepatic parenchyma was cirrhotic. Microscopically, most of the main tumor comprised multiple irregularly shaped and slightly dilated ducts (Fig. 2A). These bile ducts were surrounded by dense fibrous connective tissue and occasional lymphocytes, typical of VMCs (Fig. 2B). The lining epithelium was a single layer of flattened cuboidal-to-columnar cells (Fig. 2C). The surrounding liver was affected by sweeping fibrosis, resulting in jigsaw-like micronodules, consistent with biliary cirrhosis (Fig. 2D). The epithelial cells possessed amphophilic cytoplasm and a basal round-to-ovoid nucleus.
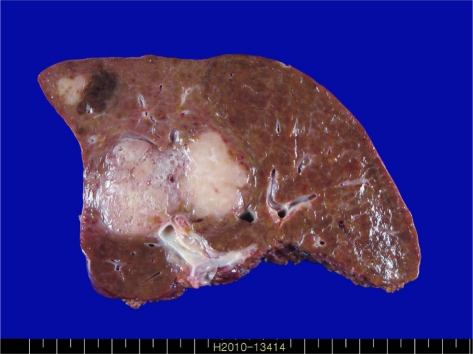
Macroscopic findings of the right lobe of the liver. The cut surface exhibits an ill-defined mass intermingled with cystic and solid areas and measuring 7.0×6.5×5.0 cm, and another white nodule, located in the subcapsular region, measuring 1.5×1.5×1.0 cm.
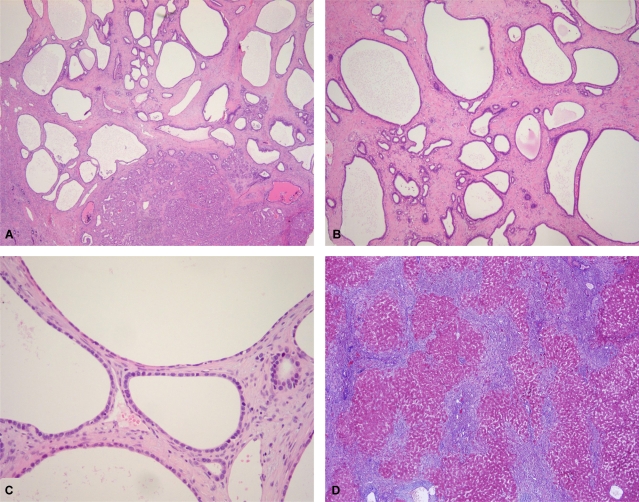
Microscopic findings of von Meyenburg complexes (VMCs) and nonneoplastic liver tissue. Microscopically, the main tumor consists of a solid part (bottom right) and multiple irregularly shaped and slightly dilated ducts (A). These bile ducts are surrounded by dense fibrous connective tissue and occasional lymphocytes, typical of VMCs (B). The lining epithelium of the cysts is a single layer of multifocally flattened cuboidal-to-columnar cells (C). The nonneoplastic liver contains jigsaw-like micronodules separated by sweeping fibrosis, comparable to biliary cirrhosis (D) (A: hematoxylin and eosin (H-E), ×20; B: H-E, ×40; C: H-E, ×200, D: Masson's trichrome, ×40).
Multiple foci of dysplastic epithelium were found among the dilated bile ducts. These contained multiple regions of pseudostratified cells with enlarged nuclei and some apical snouts. Individual sections of dysplastic epithelia were hyperchromatic and had nuclei of various sizes with loss of polarity (Fig. 3A). Well to moderately differentiated infiltrative glandular or solid structures with desmoplastic stromal reactions were also evident (Fig. 3B). Perineural invasion was present (Fig. 3C). Mitotic figures were seen occasionally, but no necrosis was observed. The characteristics of another mass was microscopically consistent with those of VMCs. The remaining liver parenchyma exhibited micro-/macronodular cirrhosis. Immunohistochemically, the VMCs, dysplastic foci, and carcinoma were positive for cytokeratin (CK)7 and CK19. The tumor cells were negative for hepatocyte antigen and CK20 staining. The carcinomatous area was focally positive and the VMCs were negative for p53. The Ki-67 labeling index was higher in solid areas of the CC exhibited than in the VMC and transitional areas (Fig. 3D).
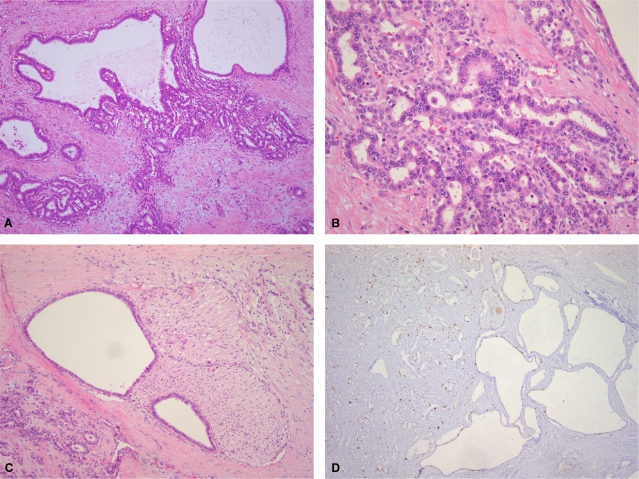
Microscopic findings of cholangiocarcinoma. In the transitional area, pseudostratification, epithelial tufting with mild-to-moderate nuclear pleomorphism, and hyperchromasia are observed in the cysts (A). Well-to moderately differentiated cholangiocarcinoma with desmoplastic stromal reactions is evident in the solid area (B). Perineural invasion is present (C). The Ki-67 labeling index is higher in the solid cholangiocarcinoma area than in the VMC and transitional areas (D) (A: H-E, ×100; B: H-E, ×200; C: H-E, ×100, D: Ki-67, ×40).
DISCUSSION
VMCs were first described by von Meyenburg in 1918 as benign malformations of the hepatobiliary system. They may be found as isolated or multiple lesions. The nodular lesions vary in size from 0.1 to 1.5 cm in diameter, but lesions with diameters larger than 3 cm have also been described.9 It can be argued that patients who have a large VMC or multiple VMCs and who are without life-threatening sequelae will have a correspondingly higher chance of developing hepatic carcinoma. However, few studies have found an association between the size or number of VMCs and the development of hepatic malignancy. Song et al suggest that the size of VMCs is a major factor in malignant transformation, and thus recommend regular follow-up to monitor any size increases in cases of large VMCs.7 The present case presented with a 7.0-cm-sized solid, cystic mass, and the VMCs were larger than typical VMCs. Preoperatively, it was necessary to distinguish VMCs from other hepatic lesions such as metastatic liver tumor, true cysts, Caroli's disease, cystadenoma, cystadenocarcinoma, and liver abscess using radiologic techniques. However, the definitive diagnosis of VMCs can be made only after histological examination.
The theory regarding the pathogenesis of VMCs postulates the presence of an arrest or disruption of hepatic ductal plate remodeling during the late phase of development of the intrahepatic biliary tree.10 A malignant tumor arising from a preneoplastic lesion can be defined by observing a transition from the preneoplastic lesion to malignancy. For example, severe epithelial dysplasia frequently antedates the appearance of cancer in long-term cigarette smokers and patients with Barrett's esophagus. In the present case, transitional foci showing varying degrees of dysplasia and representing the progression from VMCs to malignant tumor were observed adjacent to CC foci. These observations indicate that VMCs may be a premalignant lesion of CC.
The malignant transformation of VMCs to intrahepatic CC remains controversial.4,5,10 Burns et al reported that long-standing cholestasis, dilatation, and hepatotoxin might play a role in bile duct carcinogenesis in patients with VMCs,5 and Jain et al provided evidence of the neoplastic transformation of VMCs.4 Since there are only a few reports in the literature documenting the existence of an association between VMCs and hepatic malignancy, further examination is needed to prove bile duct carcinogenesis in VMCs.
The significant increase in the Ki-67 labeling index from benign VMCs through to hyperplasia or dysplasia and adenocarcinoma indicates that this index represents a useful adjunct for detecting preneoplastic lesions and malignant transition in VMCs.7 In the present case, the Ki-67 labeling index was higher in solid areas of the CC than in the VMC and transitional areas.
In summary, our case showed that intrahepatic CC occurring in association with VMCs develops either via an architectural transition of benign to dysplasia or carcinoma in situ, then to malignant glands of the biliary epithelium. This suggests that VMCs are associated with an increased risk of CC of the liver. However, confirmation that VMCs are precancerous lesions of intrahepatic CC requires the careful evaluation of a large number of patients.