Serum cystatin C level is a useful marker for the evaluation of renal function in patients with cirrhotic ascites and normal serum creatinine levels
Article information
Abstract
Background/Aims
Several studies suggested that serum cystatin C (CysC) is more useful than serum creatinine (Cr) for the assessment of renal function in patients with liver cirrhosis. This study evaluated the clinical significance of CysC in patients with cirrhotic ascites and normal Cr level.
Methods
We enrolled patients with cirrhotic ascites and a normal serum Cr level (<1.2 mg/dL). GFR was measured by 99mTc-DTPA renal scan. Serum Cr, CysC, and Cr clearance (CCr) were measured on the same day. Significant renal impairment and severe renal impairment were defined as GFR <60 mL/min and GFR <30 mL/min, respectively.
Results
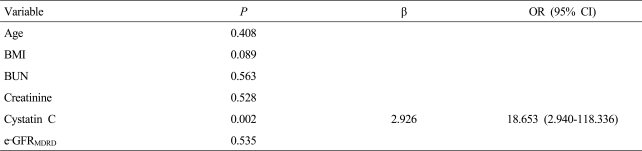
Eighty-nine patients with cirrhotic ascites were enrolled in the study (63 men and 26 women; age, 55±11 years). Forty-seven (52.8%) and 42 (47.2%) patients were in Child-Pugh grade B and C, respectively. Serum Cr and CysC levels and GFR were 0.8±0.2 mg/dL, 1.1±0.3 mg/L, and 73.4±25.5 mL/min, respectively. Significant and severe renal impairment were noted in 28 (31.5%) and 2 (2.2%) patients, respectively. GFR was well correlated with serum Cr, CysC, and e-GFRMDRD, while it was not correlated with e-GFRC&G. In multivariate analysis, only CysC was significantly correlated with GFR (β, 45.620; 95% CI, 23.042-68.198; P<0.001). Serum CysC level was the only independent predictor for significant renal impairment.
Conclusions
Significant renal dysfunction was not rare in patients with cirrhotic ascites, even their serum Cr level is normal. Serum CysC is a useful marker for detecting significant renal dysfunction in these patients.
INTRODUCTION
Renal dysfunction is common in patients with liver cirrhosis, which occurs about 19% of hospitalized patients with cirrhosis,1 due to several reasons as follows: cirrhotic patients tend to be intravascular volume depletion state due to gastrointestinal bleeding, diuretics use, and lactulose-induced diarrhea. Furthermore, these patients are often exposed to nephrotoxic agents such as nonsteroidal anti-inflammatory drugs, contrast agents, and aminoglycoside. In addition, renal dysfunction usually progresses to hepatorenal syndrome (HRS) with progression of liver cirrhosis and portal hypertension.2 Patients with cirrhosis who develop HRS have very high mortality, and even with terlipressin and albumin only 40% respond and survive for 1 month after treatment.3 Therefore, because renal dysfunction is directly linked to the mortality rate of cirrhotic patients, a precise assessment of renal function is required to estimate the prognosis and determine the correct therapeutic intervention and response.
Serum creatinine (Cr) is commonly used marker for the assessment of renal function in the general population. However, serum Cr level could be influenced by age, gender, ethnicity, protein intake, and muscle mass.3 Serum Cr may overestimate renal function, especially in patients with liver cirrhosis. Impaired liver function, protein-calorie malnutrition and muscle wasting result in decreased Cr production.4,5 Elevated serum bilirubin can interfere with the measurement of Cr by Jaffe method. In addition, ascites and peripheral edema can also decrease the Cr by widening the distribution of Cr in the body. Therefore, baseline serum Cr is low in cirrhotic patients compared to general population.6,7 It has been suggested that many patients with cirrhosis and ascites will have a low glomerular filtration rate (GFR) despite normal serum Cr level.8 The Cockcroft and Gault and the modification of diet in renal disease (MDRD) equations are widely used in the general population to estimate GFR.6,7 However, because these equations are based on serum Cr levels, they are also inaccurate in cirrhotic patients.
Recently, it was reported that serum cystatin C (CysC) can be used alternative to serum Cr.9 CysC is a low molecular weight protein produced at a constant rate by all nucleated cells and eliminated by glomerular filtration.10 After filtration CysC is reabsorbed and catabolized by the tubular epithelial cells. In contrast to Cr, CysC is independent of gender, age, and muscle mass. The dosage is not influenced by serum bilirubin, inflammation, or malignancy.3,11 In a recent metaanalysis, serum CysC was superior to serum Cr and had better correlation with GFR.12 In a previous study, serum CysC was at least as accurate as serum Cr in patients with HRS.13 Several reports have suggested that increased serum CysC levels are more sensitive for detecting renal dysfunction in patients with cirrhosis than increased serum Cr levels, and that measurement of serum CysC could offer good alternative to serum Cr for the assessment of renal function in these patients.11,13-15
This study was performed to evaluate the frequency of renal dysfunction in patients with cirrhotic ascites and a normal serum Cr level and the clinical significance of CysC for the detection of the renal dysfunction in these patients.
PATIENTS AND METHODS
Patients
Consecutive patients with cirrhotic ascites and a normal serum Cr level (<1.2 mg/dL) who were admitted to the Korea University Anam Hospital between January 2008 and December 2009 were enrolled in this study. Patients with hepatocellular carcinoma, intrinsic renal disease, congestive heart failure, chronic obstructive pulmonary disease, spontaneous bacterial peritonitis, severe malnutrition, sepsis or gastrointestinal bleeding during the month before enrollment were excluded from the study. The diagnosis of cirrhosis was based on a combination of physical examination, laboratory tests, and imaging. Informed consent was obtained from each patient according to the Declaration of Helsinki, and the study protocol was approved by the local ethics committee at our hospital.
Laboratory analyses
Serum samples were obtained for the measurement of Cr, blood urea nitrogen (BUN) and CysC. Serum Cr levels were determined using the Dimension clinical chemistry system (Dade Behring, Marburg, Germany) with a commercially available assay based on the modified Jaffe method, as reported by Larsen.16 The serum CysC assay was implemented using latex-particle-enhanced turbidimetric immunoassay PET (Dako, Glostrup, Denmark) on a CX7 analyzer (Beckman, CA, USA). The Dako Cystatin C PET kit contains polystyrene particles of uniform size that were chemically coupled to rabbit antibodies raised against human CysC. Biochemical tests, including for levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, albumin and bilirubin, were implemented using routine laboratory methods. The Child-Pugh score was determined by applying Pugh's commonly used modification, which is based on the presence and severity of ascites and hepatic encephalopathy, prolongation of the prothrombin time and levels of serum bilirubin and albumin.17 The model for end-stage liver diseases (MELD) score was calculated according to the following equation: 9.57×loge (creatinine, mg/dL)+3.78×loge (bilirubin, mg/dL)+11.20×loge (INR)+6.43, where INR is the international normalized ratio and 6.43 is the constant for liver disease etiology.18 The minimum value was set at 1.0 for calculation purposes. The maximum serum Cr level considered in the above equation was 4.0 mg/dL.
GFR was measured by 99mTc-DTPA renal scan. Images were obtained in supine position following an intravenous injection of 99mTc-DTPA with a dose of 300 MBq. The data were acquired with a large field of view gamma camera equipped with a low energy, parallel hole general purpose collimator and an acquisition matrix. The first 60 s of the dynamic acquisition used a frame rate of 2 s/frame followed by a frame rate of 45 s/frame for a total duration of 20 minutes. An image of glomerular filtration was generated on a pixel-by-pixel basis.19 The basis of the filtration image is based on the kidney's ability to take up the tracer from the blood, the image is independent of the volume of distribution of the tracer and any edema that may be present.
Significant renal impairment and severe renal impairment were defined as GFR <60 mL/min and GFR <30 mL/min, respectively, according to the National Kidney Foundation guidelines.20 In addition, two kinds of estimated GFR (e-GFR) were calculated: (i) using the formula of Cockcroft and Gault (e-GFRC&G)6 and (ii) using the MDRD equation (e-GFRMDRD).7 Creatinine clearance (CCr) was calculated as a product of urinary Cr and 24-h urine volume divided by serum Cr (mg/dL) and multiplied by 1440.
Statistical analysis
Statistical analysis was performed using SPSS version 13.0 (SPSS, Chicago, IL, USA). All data are expressed as either mean±standard deviation (SD) values or the number of patients (as a percentage of the entire cohort). Since distributions of continuous data were skewed, nonparametric methods were used for the group comparison and correlation analyses. Qualitative and quantitative differences between subgroups were analyzed using the χ2 test and the Mann-Whitney U-test, respectively. Spearman's correlation analysis was used to assess relationships between GFR and Cr, CysC, CCr, e-GFRC&G, e-GFRMDRD. Binary logistic regression analyses were performed for detection of patients with significant renal impairment. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to determine the level of significance in the multivariate analysis. The predictive efficacy of a variable for predicting significant renal impairment was evaluated using a receiver operating characteristic (ROC) curve, with the area under the ROC curve (AUC) and 95% CIs used as indices of accuracy. The optimal cutoff value for predicting significant renal impairment was determined based on the maximum total sensitivity and specificity. The cutoff for statistical significance was set at P<0.05.
RESULTS
Baseline characteristics
The baseline characteristics of the 89 enrolled patients with cirrhotic ascites are presented in Table 1. The age was 55±11 years and 63 patients (70.8%) were male. Disease etiologies comprised alcohol (n=40, 44.9%), chronic hepatitis B (n=28, 31.5%), chronic hepatitis C (n=7, 7.9%), autoimmune liver disease (n=5, 5.6%), and cirrhosis of unknown etiology (n=9, 10.1%). Forty-seven (52.8%) and 42 (47.2%) patients were in Child-Pugh class B and C, respectively. Serum Cr and CysC levels and GFR were 0.8±0.2 mg/dL, 1.1±0.3 mg/L, and 73.4±25.5 mL/min, respectively. Significant renal impairment and severe renal impairment were noted in 28 (31.5%) and 2 (2.2%) patients, respectively.
Correlation of the variables with GFR
GFR was well correlated with 1/Cr (Spearman's coefficient, 0.242; P=0.022) and 1/CysC (0.387; P<0.001), and e-GFRMDRD (0.302; P=0.004), while it was not correlated with e-GFRC&G (-0.086; P=0.422) (Fig. 1). In multiple linear regression analysis, only 1/CysC was significantly correlated with GFR (β, 45.620; 95% CI, 23.042-68.198; P<0.001) (Table 2).
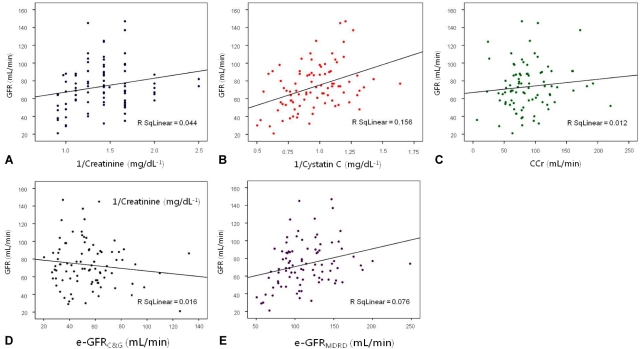
Scatter plots of glomerular filtration rate (GFR) versus serum creatinine (A) and cystatin C (B) levels, creatinine clearance (C), e-GFRC&G (D), and e-GFRMDRD (E).
CCr, creatinine clearance; e-GFRC&G, glomerular filtration rate as estimated using the formula of Cockcroft and Gault; e-GFRMDRD, glomerular filtration rate as estimated using the modification of diet in renal disease equation.
Detection of significant renal impairment
Age, serum Cr and CysC levels, and e-GFRMDRD were significantly different between patients with significant renal impairment and the others (Table 3). However, serum CysC level was the only independent factor for predicting significant renal impairment (Table 4).
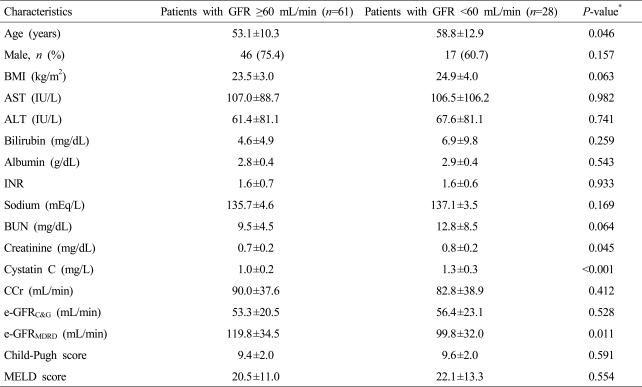
Baseline characteristics of enrolled patients with cirrhotic ascites according to their renal function
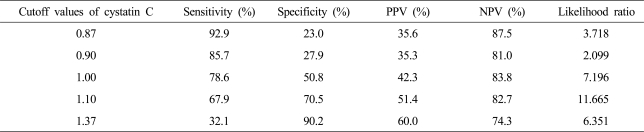
AUCs for predicting significant renal impairment of serum Cr and CysC levels, CCr, e-GFRC&G, and e-GFRMDRD were 0.615 (95% CI, 0.478-0.751), 0.721 (0.602-0.841), 0.561 (0.430-0.691), 0.463 (0.331-0.594), and 0.659 (0.529-0.788), respectively (Fig. 2). Sensitivity, specificity, PPV, and NPV for predicting significant renal impairment according to the cutoff values of serum CysC level are presented in Table 5. Optimal cutoff value of serum CysC level was set at 1.1 mg/L. Serum CysC level was ≥1.1 mg/L in 37 patients (41.6%) and GFR was <60 mL/min in 19 of them (67.9%).
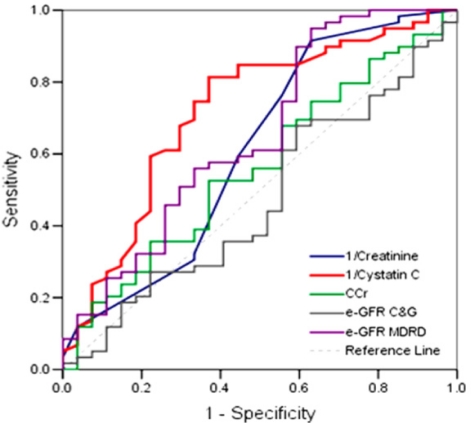
Receiver operating characteristic curves of serum creatinine and cystatin C levels, CCr, e-GFRC&G, and e-GFRMDRD for predicting significant renal impairment.
CCr, creatinine clearance; GFR, glomerular filtration rate; e-GFRC&G, glomerular filtration rate as estimated using the formula of Cockcroft and Gault; e-GFRMDRD, glomerular filtration rate as estimated using the modification of diet in renal disease equation.
DISCUSSION
Many patients with liver cirrhosis and ascites will have a lower GFR but a normal serum Cr level.8 In these patients, renal dysfunction usually progresses with liver cirrhosis and portal hypertension.21 HRS is a functional renal failure that frequently develops in advanced cirrhotic patients. Splanchnic vasodilatation and reduced cardiac output plays a significant role for the development of HRS.21 The incidence of HRS was 18% at 1 year and 39% at 5 years in advanced cirrhosis.21 In our previous study, the cumulative incidence of HRS at 1 year of follow-up was 20.4%.22 The prognosis of HRS is very poor. The median survival was only 1.7 weeks and the mortality rates at 1 and 2 months were 75% and 82%, respectively.23 So, the development of renal dysfunction significantly affects the prognosis of the patients with cirrhosis. Several authors have suggested that renal function is better than liver function as a predictor of prognosis in these patients.3,24,25 In our study, significant renal dysfunction was noted in 28 patients (31.5%) in patients with cirrhotic ascites, even their serum Cr levels were normal.
Serum Cr has been widely used as the standard laboratory marker for the assessment of renal function in general population. However, serum Cr could not represent GFR in several conditions, especially in liver disease.8,26,27 Serum Cr is influenced by age, gender, ethnicity, muscle mass and protein intake.28 Because Cr is a byproduct of the metabolism of the nitrogenous organic acid, creatine, which is stored in muscles, Cr reflects endogenous muscle mass as well as protein intake.29 Routine Cr assay is based on spectrophotometry. In patients with jaundice, bilirubin interferes with Cr dosage as a chromogen, results in low Cr value.30,31 In addition, because creatine is synthesized in the liver, any cause of hepatic parenchymal dysfunction will directly reduce creatine production.29 Decrease in the creatine production rate as well as malnutrition and muscle wasting in patients with liver cirrhosis lead to a markedly lower baseline Cr compared to the normal population.32,33 Therefore, serum Cr may overestimate renal function in these patients and normal serum Cr level cannot exclude early renal dysfunction in patients with liver cirrhosis.8,26,34,35
CCr from timed urine collections might be a reliable method for renal function evaluation. However, several studies have shown that CCr overestimates true GFR about 13 mL/min/1.73 m3 compared to inulin clearance in patients with cirrhosis,14,35-37 because the increased proportion of Cr secreted by the tubule compared to Cr filtered by the glomerulus in these patients.36 Practically, nonspecific factors including incomplete urine collection due to hepatic encephalopathy and errors in the timing of collection may play a role in the inaccuracy of CCr. Consistently, CCr was not correlated with GFR (P=0.228) in this study.
The Cr-based e-GFRC&G and e-GFRMDRD are commonly used method to estimate GFR. However, as these equations are based on serum Cr, they are also inaccurate in patients with cirrhosis. Several studies have reported that both e-GFRC&G and e-GFRMDRD tend to overestimate true GFR.26,34,36,38 The inaccuracy of e-GFRC&G and e-GFRMDRD in cirrhotic patients may be related to several factors. At first, Cr is inaccurate marker of renal function in cirrhotic patients. Secondly, e-GFRC&G and e-GFRMDRD include serum Cr adjusted for several variables which were shown to have a significant impact on GFR in the general population (i.e., age, body weight and gender for e-GFRC&G; age, gender and ethnicity for e-GFRMDRD). These factors did not fit for cirrhotic patients. Finally, e-GFRC&G and e-GFRMDRD are not adjusted for some variables which are likely to have a determinant impact on the estimation of GFR in cirrhotic patients. e-GFRC&G relies on the serum Cr as well as the body weight which is difficult to define in patients with ascites. This formula has a sensitivity of only 50-60% for detecting a loss of GFR in patients with liver disease.39 In cirrhotic patients, e-GFRMDRD is relatively more accurate than e-GFRC&G because it does not consider body weight.40 Consistently, GFR was significantly correlated with e-GFRMDRD, while not with e-GFRC&G in this study.
In this study, 1/Cr, 1/CysC, e-GFRMDRD, was well correlated with GFR in a univariate analysis, but only 1/CysC was significantly correlated with GFR in a multivariate analysis. The strength of this study is that we compared Cr, CysC, and CCr with directly measured GFR using 99mTc-DTPA scan, rather than estimated GFR based on serum Cr level. However, it should be kept in mind that inulin clearance is still considered as gold standard for determination of GFR, although it is impractical because of the necessity for a continuous intravenous infusion and urine bladder catheter for urine collections over a period of several hours. In contrast, because radiolabeled tracers such as 99mTc-DTPA, 51Cr-EDTA, and 125I-iothalamate require only a single injection and timed urine collection is not needed, these examinations could be more practical. Because 99mTc-DTPA is filtered by the glomerulus, and neither reabsorbed nor excreted by the tubules, 99mTc-DTPA renal scan offers the possibility to estimate both renal perfusion and glomerular filtration.41 A 20 minutes 99mTc-DTPA renal scan with a gamma camera allows semi-quantitative analysis of the perfusion and filtration phases but absolute quantification is fraught with difficulty on the gamma camera.41 In addition, GFR obtained by these examinations may overestimate compared to GFR obtained by inulin clearance.42,43 Usually, all plasma-clearance techniques become somewhat inaccurate in conditions with GFR values below 20-30 mL/min.44
We had expected that serum CysC level could be a good marker of renal dysfunction in patients with normal serum Cr level in cirrhotic patient with ascites. In accordance with our expectation, serum CysC was a good marker of early renal dysfunction in patient with cirrhotic ascites and normal Cr levels. The cutoff value of 1.1 mg/dL could be an adequate reference level for detecting early renal dysfunction in these patients. In this study, there was no significant difference in MELD and Child Pugh score between normal and impaired renal function group, probably because we enrolled advanced cirrhosis patients who already have decompensated cirrhosis with ascites. Our results were consistent with previous studies.13,15,45-47 However, evaluation of renal function using serum CysC levels has several limitations. Firstly, CysC assay is more expensive than serum Cr assay. Secondly, the assays need more standardization.48 Lastly, serum CysC is influenced by infection and some drugs such as corticosteroids, angiotensin-converting enzyme inhibitors or calcineurin inhibitors (CNI).49,50
In conclusion, significant renal dysfunction is not rare in patients with cirrhotic ascites, even their Cr level is normal. Serum CysC could be a useful marker for detecting significant renal dysfunction in these patients.
Acknowledgements
This work was supported by The GlaxoSmithKline Research Fund of the Korean Association for the Study of the Liver and by a grant from the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (No. A050021).
Abbreviations
ALT
alanine aminotransferase
AST
aspartate aminotransferase
BMI
body mass index
BUN
blood urea nitrogen
C&G
the Cockcroft and Gault equation
CCr
creatinine clearance
Cr
creatinine
CysC
cystatin C
GFR
glomerular filtration rate
HRS
hepatorenal syndrome
INR
international normalized ratio
MDRD
the modification of diet in renal disease equation
MELD
model for end-stage liver diseases