A case of hepatocellular carcinoma in the caudate lobe successfully treated by transcatheter arterial chemoembolization using drug-eluting beads
Article information
Abstract
Hepatocellular carcinoma (HCC) in the caudate lobe remains one of the most intricate locations where various treatments tend to pose problems with regard to the optimal approach. Surgical resection has been regarded as the most effective treatment; however, isolated resection of the caudate lobe is strenuous and associated with a high rate of early recurrence. Percutaneous ablation might be technically difficult or impossible to perform due to the deep location of tumors and adjacent large vessels. Treatment with drug-eluting beads (DEB) can potentially enhance the therapeutic efficacy for patients with unresectable HCC by drawing on the slower, more consistent drug delivery process. We described a case of a 62-year-old man with HCC in the caudate lobe who was successfully treated by DEB.
INTRODUCTION
Hepatocellular carcinoma (HCC) is a common tumor with a poor prognosis especially in the hepatitis B endemic country such as Korea. Surgical resection, liver transplantation, and percutaneous ablation are generally accepted as the only curative treatments for HCC.1 Transcatheter arterial chemoembolization (TACE) is the standard first-line treatment for patients with inoperable and intermediate HCC, but response rates and evidence of a survival benefit is relatively low.2 Although there are several therapeutic options for the treatment of HCC, HCC in the caudate lobe is regarded as a clinical hot potato owing to limitation of applicable therapeutic method pertinent to the lesion.
Recently, drug delivery embolization system, that is drug-eluting beads (DEB), with the ability to sequester chemotherapeutic agent from solution and release it in a sustained and controlled mode were introduced for TACE.3 We described a case of early-staged caudate HCC which was treated completely by TACE using DEB.
CASE REPORT
A 62-year-old man with alcoholic cirrhosis visited outpatient clinic for unexplained weight loss. He had a good performance status (grade 0). Laboratory tests showed serum albumin level of 5.3 g/dL, bilirubin level of 1.3 mg/dL, and prothrombin time international normalized ratio of 1.1. Neither ascites nor hepatic encephalopathy was found, which corresponded with Child-Pugh class A. Serologic markers for hepatitis B and C were negative. Serum α-fetoprotein level was 311.7 ng/mL, and PIVKA-II level was over 2,000 mAU/mL. Other laboratory findings including tumor markers and thyroid function tests were within normal limits. About a 5 cm sized ovoid enhancing mass in arterial phase involving the caudate lobe with underlying cirrhotic configuration of the liver was shown on the dynamic computed tomography (CT) and magnetic resonance imaging (MRI) (Fig. 1A-B and 1C-D, respectively). There was no vascular invasion and no intraabdominal lymphadenopathy. On positron emission tomography-CT, maximum standardized uptake value for the mass was 6.3 without evidence of distant metastasis. Overall, early stage of HCC was assigned according to the Barcelona-Clinic-Liver-Cancer (BCLC) HCC staging system.1
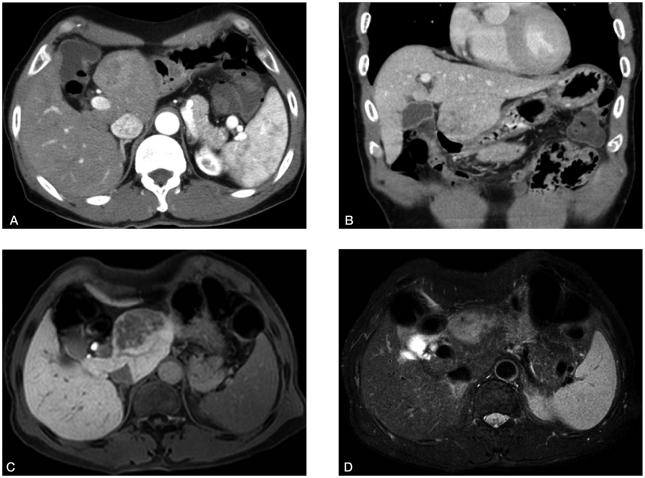
An irregularly enhanced mass measuring 5 cm in the caudate lobe with delayed wash-out was observed on dynamic CT (A: arterial phase, B: portal phase, coronal section). Similar signal intensity was noted on MRI (C: T1-weighted image, D: T2-weighted image).
Surgical resection was recommended for the treatment of the HCC, however, he refused the surgical treatment. TACE using DEB was performed after obtaining written informed consent for the procedure. During the first TACE session, hepatic angiogram revealed a definite hypervascular mass supplied by a small branch of the left hepatic artery without apparent arteriovenous shunt (Fig. 2A-B). After positioning the microcatheter tip at the left hepatic artery close to the tumor-feeding branch, a mixture of DEB (DC Bead®, 1 vial of 300~500 µm diameter, Biocompatibles, UK) loaded with doxorubicin (50 mg) and contrast agent was infused until the flow through the tumor-feeding artery was slowed down. After the infusion, angiogram showed occluded tumor-feeding branch and no tumor staining (Fig. 2C).
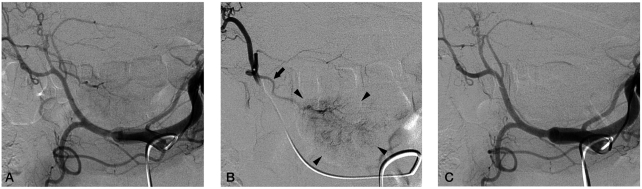
(A) Celiac arteriography showed a faint hypervascular tumor staining in the caudate lobe supplied by a branch from the left hepatic artery. (B) Selective left hepatic arteriography clearly demonstrated the tumor (arrow heads) and a single supplying artery branch (arrow) from the left hepatic artery. (C) Post-embolization celiac arteriography showed no more tumor staining and occlusion of the tumor supplying artery.
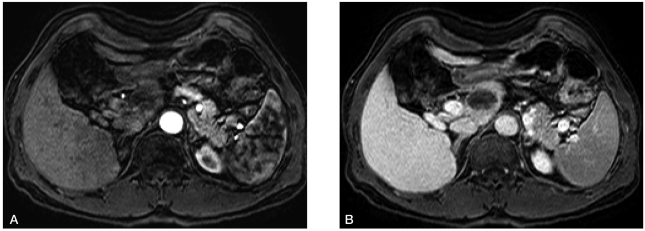
He had no serious adverse event after embolization except mild abdominal pain that was controlled with oral analgesic (tramadol 50 mg per os tid). One-month post-embolization CT (Fig. 3A-B) and six-month post-embolization MRI (Fig. 4A-B) showed complete necrosis without viable portions. Follow-up α-fetoprotein level was 3.89 ng/mL, and PIVKA-II level was 21 mAU/mL. This patient remains in good health without cancer recurrence 6 months after the procedure.

Complete necrosis of a previously noted lesion without remnant or new enhancement was observed (A: arterial phase, B: portal phase).
DISCUSSION
Although HCC arising in the caudate lobe is no common in clinical setting, it is problematic due to its deep anatomical location and close relationship with major vasculatures. Caudate lobe occupies the space below the hepatic hilum in close proximity to ductal and portal confluences and the proximal hepatic arteries, above the inferior vena cava.4 Frequent invasion of the vessels and frequent formation of tumor thrombi in portal vein and inferior vena cava might contribute to intra- and extrahepatic metastases.5 The limitations in proper therapeutic approach to the lesion is clinically challenging. For these reasons, prognosis of caudate HCC is relatively poor.5
Surgical resection has been generally regarded as curative method for HCC. Although HCC in caudate lobe is rare, resection would be the most effective treatment and provide long-term survival.6 Recently, improvement in surgical technique enables successful resection of caudate HCC, however, several operative risks and HCC recurrence are still remained problems.7-10 As an alternative treatment modality, radiofrequency ablation was conducted to treat caudate HCC by several methods, i.e. conventional percutaneous, laparoscopy-assisted, or intra-operative approaches.11-13 But the proximity to major vessels contains potential risk of complication and incomplete ablation related with heat sink effect.
In this case, patient's stage according to BCLC criteria was early, proposed to be treated effectively by resection, liver transplantation or ablation.1 Although TACE was not suitable as first-line therapy for such an early-staged patient, TACE can be performed in patients at the early stage in whom ablation cannot be performed because of tumor location or medical comorbidities.1 By reason of aforementioned treatment limitations and patient's refusal of surgery TACE using DEB was performed, and as a result, therapeutic response was satisfying without any serious complications.
DEB are a new soft deformable device of spherical shape composed by a polyvinyl alcohol and a hydrophilic monomer that can be loaded with anthracycline derivatives such as doxorubicin.14 So, drug-loaded beads can be delivered intra-arterially into the tumor and elute the chemotherapeutic effect.3 Previous studies have shown that TACE with DEB results in promising efficacy and low toxicity.15-17 Post-embolization syndrome, cholecystitis, liver abscess, and skin rash were reported as procedure-related complications.17 In recent European multicenter comparative study, 212 patients with Child-Pugh A/B cirrhosis and large and/or multinodular, unresectable HCC (BCLC classification A or B) were randomized to receive either conventional TACE or DEB-TACE. According to the per-protocol analysis, objective response rate in the DEB-TACE group was 52%. In subgroup analysis, patients with more advanced disease-defined as Child-Pugh stage B, Eastern Cooperative Oncology Group performance status 1, bilobular or recurrent disease-had a significantly greater objective response rate (53%) in the DEB-TACE group.18
We cannot assert the efficacy if the patient underwent conventional TACE instead of DEB-TACE. Terayama et al reported that cumulative local recurrence rate after subsegmental TACE for caudate HCC was very high compared with recurrence rate after subsegmental TACE for HCC in the other segments, and technical success rate of the procedure was relatively low (around 70%).19 In previous study that analyzed explants after pre-transplant conventional TACE, a total or at least subtotal necrosis (≥ 80%) was noted in 30% on the liver explants, and half of the explants showed tumor necrosis less than 50%.20 However, experimental study has shown that complete tumor destruction was observed in the DEB-treated animals sacrificed 7 days after treatment.3 It means that DEB could be a potential curative therapeutic modality for HCC if there is no problem regarding embolization procedures.
We report a case of caudate HCC successfully treated with DEB-TACE. TACE using this new agent could be an alternative therapy or downstaging method for the caudate HCC patients who are not eligible to conventional treatment including surgical resection.
Acknowledgements
The authors have nothing to disclose regarding funding or conflict of interest with respect to this manuscript.
Abbreviations
BCLC
Barcelona-Clinic-Liver-Cancer
CT
computed tomography
DEB
drug-eluting beads
HCC
hepatocellular carcinoma
MRI
magnetic resonance imaging
TACE
transcatheter arterial chemoembolization