| Korean J Hepatol > Volume 16(4); 2010 > Article |
ABSTRACT
Background/Aims
Transarterial chemoembolization (TACE) improves the survival of patients with unresectable hepatocellular carcinoma (HCC) and has been recommended as a first-line therapy for nonsurgical patients with large or multifocal HCC. The long-term outcome of HCC patients receiving TACE prior to hepatic resection is uncertain.
Methods
Between January 1997 and
December 2007, 1,530 patients underwent hepatic resection for HCC at our center. Thirty-two patients received 1~12 sessions of TACE followed by surgical resection (TACE-surgery group). Their overall and recurrence-free survival rates were compared with those of 64 age- and sex-matched controls who underwent surgery only (surgery group). Overall and recurrence-free survival rates were analyzed.
Results
The 1-, 2-, and 5-year overall survival rates did not differ significantly between the TACE-surgery group and the surgery group (78%, 60%, and 26%, respectively, vs. 97%, 83%, and 45%, respectively; P=0.11); however, the 1-, 2-, and 5-year recurrence-free survival rates were significantly lower in the TACE-surgery group than in the surgery group (58%, 36%, and 7%, respectively, vs. 77%, 58%, and 32%, respectively; P=0.01). The distribution of recurrence sites in the TACE-surgery group were intrahepatic in 85.7% and extrahepatic in 14.3%, and did not differ from those in the surgery group (91.4% and 8.6%, respectively; P=0.66).
Hepatocellular carcinoma (HCC) is the fifth most common
cancer and the third most common cause of cancer-related death in the world.1,2 For selected patients with HCC, hepatic resection is one of the most reliable curative treatments, showing a 5-year survival rate of 50% or higher.3 However, because of advanced stage of HCC and/or inadequate functional hepatic reserve, only 9~30% of newly diagnosed patients are candidates for surgical resection.3-7
Transarterial chemoembolization (TACE) involves the injection of lipiodol (iodized poppyseed oil) and a chemotherapeutic agent into the hepatic artery supplying the tumor, followed by embolization with gelatin or gelfoam particles. Recent systematic reviews have demonstrated that TACE improves survival of patients with unresectable HCC and the procedure may become one of the standard treatments for HCC.8-10 TACE has been recommended as first-line therapy for nonsurgical patients with large or multifocal HCC.11
In the past, lipiodol computed tomography (CT) taken after TACE had been one of the most useful and frequently used tools for diagnosis and precise staging of HCC before surgery. Since the introduction of advanced imaging modalities such as dynamic CT and magnetic resonance image (MRI) with hepatocyte-specific contrast media, diagnostic role of TACE is gradually decreasing. However, TACE is still necessary prior to surgical resection in a portion of HCC patients after performing all available imaging, tumor markers, and even biopsy; for accurate diagnosis and staging of intrahepatic disease, reduction in the size of the tumor to increase resectablility, or palliative control of HCC when surgery is temporarily impossible.12-14 However, long term prognosis of hepatic resection in HCC patients with preoperative TACE remains uncertain.14-19
Hence, we compared the outcome of HCC patients who underwent surgical resection following various sessions of preoperative TACE with that of patients who underwent surgery without any preoperative treatment, focusing on overall and recurrence-free survival and patterns of recurrence.
From January 1997 to December 2007, 1,530 patients underwent hepatic resection for HCC at our center. Of these, 32 patients underwent one or more sessions of TACE and no other treatment for HCC before surgical resection (TACE-surgery group). Among patients who did not receive any kind of treatment for their HCC prior to surgery, 64 age- and sex-matched patients were chosen as controls using a random number table (surgery group). Patients who underwent other forms of treatment such as radiofrequency ablation before surgery or those who received liver transplantation were excluded.
The diagnosis of HCC was based on histological criteria and/or imaging techniques and alpha-fetoprotein (AFP) levels, as proposed by the Korean Liver Cancer Study Group and the National Cancer Center.20,21 HCC was initially diagnosed for a combination of AFP > 400 ng/mL and a positive arterial image, or AFP < 400 ng/mL and positive arterial images from at least two modalities. All tumors were consequently confirmed as HCC by histological examination of surgical specimens. Tumors were staged according to the modified UICC classification system by Korean Liver Cancer Study Group.21
Patients in the TACE-surgery group received 1-12 sessions of TACE [2.6±2.1, mean±standard deviation (SD)] prior to resection. Main reasons for preoperative TACE were confirmation of the diagnosis and staging of liver lesions (n=18, 46.3%), down-staging of tumors (n=9, 28.1%), and palliative control of HCC when surgery is temporarily impossible due to patients' refusal (n=4, 12.5%) or recent traffic accident (n=1, 3.1%). Surgery was done 102.9±130.7 days (mean±SD) after last TACE in TACE-surgery group. Interval from the diagnosis of HCC to operation was 322.7±403.6 days (mean±SD) in TACE-surgery group and 18.5±12.4 days (mean±SD) in surgery group (P<0.001).
None of the patients received post-operative adjuvant treatment. The routine post-operative follow-up protocol included biochemical liver function tests, serum AFP concentrations, and triphasic dynamic CT scan performed every 3 months during the first 2 years after surgery, and every 4~6 months thereafter.
A vascular catheter was inserted through the femoral artery, and hepatic angiography was performed. After assessing the hepatic vascular anatomy, TACE was performed through lobar or segmental hepatic arteries. In all patients, TACE was performed with a mixture of iodized oil (Lipiodol; Guerbet, Aulnay-sous-Bois, France) and doxorubicin hydrochloride (Adriamycin; Kyowa Hakko Kogyo, Tokyo, Japan) at a dose of 3 mg doxorubicin hydrochloride and 1 cc iodized oil per 1 cm tumor diameter. Gelfoam powder (Upjohn, Kalamazoo, MI, U.S.A) was used for particle embolization. The injection was continued until stasis was identified in the feeding artery.
Baseline characteristics of patients and tumors were evaluated in the TACE-surgery group and surgery group. Overall and recurrence-free survival rates and sites of recurrence after resection were compared between the two groups.
Categorical variables were summarized as frequency and percentage while continuous variables were presented as mean±SD. In order to compare TACE-surgery group and surgery only group, the Fisher's exact tests or chi-square tests were performed for categorical variables and the Mann-Whitney tests were used for continuous variables. Overall and disease free survival curves were estimated using a Kaplan-Meier method. These curves were compared between TACE-surgery group and surgery only group by a log-rank test. All data analyses were performed using statistical package SAS Enterprise Guide 3.0 (SAS Institute Inc., Cary, NC, USA). P<0.05 was considered statistically significant.
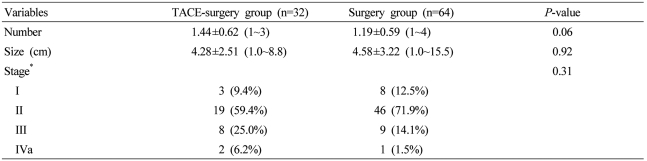
The TACE-surgery group consisted of 25 males (78.1%) and 7 females (21.9%) aged 52.4±9.8 years. Twenty-seven patients (84.4%) were HBV-positive and 30 patients (93.8%) had liver function corresponding with Child-Pugh class A. The serum AFP level was 9,584.4±26,238.5 ng/mL. All these characteristic of the TACE-surgery group were similar to those of the surgery group (Table 1).
As for tumor characteristics, the numbers of tumors in the TACE-surgery group were one in 20 patients (62.5%), two in 10 patients (31.3%), and three or more in 2 patients (6.2%). The tumor size was 4.3±2.5 cm. Modified UICC stage in the TACE-surgery group were as follows; stage I in 3 patients (9.4%), II in 19 patients (59.4%), III in 8 patients (25.0%) and IVa in 2 patients (6.2%). All these tumor characteristics were not significantly different between the TACE-surgery group and the surgery group (Table 2).
During a follow-up period of 3.2~123 months (37.3±7.7), overall and recurrence-free survival rates after resection were compared between the two groups. The 1-, 2-, and 5-year overall survival rates of the TACE-surgery group were not significantly different from those of the surgery group (78%, 60%, and 26% vs. 97%, 83%, and 45%, P=0.11; Fig. 1). Subgroup analysis according to tumor stage showed that the 1-, 2-, and 5-year overall survival rates of the TACE-surgery group were not significantly different from those of the surgery group (78%, 60%, and 37% vs. 98%, 83%, and 47%, P=0.35 in stage I-II; 70%, 54%, and 0% vs. 84%, 75%, and 17%, P=0.36 in stage III-IVa). However, the 1-, 2-, and 5-year recurrence-free survival rates of the TACE-surgery group were significantly lower than those of the surgery group (58%, 36%, and 7% vs. 77%, 58%, and 32%, respectively, P=0.01; Fig. 2). Hence, the 1-, 2-, and 5-year cumulative recurrence rates in the TACE-surgery group were 48%, 66%, and 77%, respectively, significantly higher than those in the surgery group (29%, 46% and 55%, respectively; P=0.04; Fig. 3). Distribution of recurrence sites in the TACE-surgery group were intrahepatic in 85.7% and extrahepatic in 14.3%, similar to those of the surgery group (91.4% and 8.6%, respectively; P=0.66; Table 3).
Although preoperative TACE still forms a part of current clinical practice for HCC, long term outcome after surgical resection in those patients remains controversial. Whereas several previous reports have shown that preoperative TACE is effective in reducing the incidence of postoperative recurrence and prolonging survival,14-16 other studies have failed to show a positive effect of preoperative TACE.17-19,22-26 These contradictory findings come from differences in study design, patient characteristics, stage of HCC, method and number of sessions of TACE, surgical technique, peri- and post-operative care and so on.
In this study, we investigated the influence of preoperative TACE in patients undergoing liver resection for HCC who were enrolled over a recent 10-year period in a single center so that the confounding effects of different TACE methods, surgical protocols, and post-operative management could be minimized. Sixty-four age- and sex-matched patients were randomly chosen as controls from patients who did not receive any kind of treatment for their HCC prior to surgery during the same time period. Our results suggest that HCC patients who underwent TACE before resection had overall survival rates comparable to those without pre-operative therapy, but recurrence rates were higher in patients who received preoperative TACE. In addition, preoperative TACE does not appear to promote extrahepatic tumor recurrence.
In our HCC patients with preoperative TACE, overall survival after resection was comparable to the control group without any treatment prior to surgery. A previous retrospective analysis of the outcomes of 120 patients with HCC who underwent hepatectomy with or without preoperative TACE reported cumulative 1-, 3-, and 5-year survival rates of 87%, 50%, and 32%, respectively, for all patients, with no significant differences between patients with or without preoperative TACE.15 Another retrospective study investigating the role of preoperative TACE for resectable HCC demonstrated that, in a TACE subgroup with tumor stage III-IV, preoperative TACE was supposed to reduce tumor recurrence and confer a survival advantage after surgery.16 Induction of tumor down-staging or complete necrosis by TACE was suggested to be associated with improved disease free survival.14 On the contrary, randomized prospective studies with results against the benefits of preoperative TACE were performed mostly in patients with initially resectable HCC.17-19 Poor outcome after resection in the TACE group was reported in patients without cirrhosis or those with stage I-II tumors.19 Longer operation time and/or technical difficulties during surgery, increased liver failure and gastrointestinal bleeding, and increased extrahepatic metastasis were shown to be associated with poor outcome after resection in patients with preoperative TACE.18,22,23
Our study demonstrated that recurrence-free survival rates in the TACE-surgery group were significantly lower than those in the surgery group. While improved tumor-free survival was demonstrated in patients with preoperative TACE for HCC larger than 8 cm15 and with advanced stage in one study,16 most other studies have reported similar or lower disease-free survival rates in patients receiving preoperative TACE.17-19,22 Especially, increased rate of recurrence, including extrahepatic metastasis, was reported in patients with initial resectable HCC22 or stage I-II HCC19 who received preoperative TACE. It has been suggested that TACE-induced necrosis might result in hematogenous dissemination from the primary tumor.24 The higher incidence of pulmonary metastasis following TACE in patients with HCC has been reported; however, the subjects who developed lung metastasis in that series were followed for a longer period than those without metastasis.25 Notably, a report suggested that preoperative TACE reduced the dissemination of tumor cells in the circulating blood during surgery.26 Our study also shows similar risk for extrahepatic recurrence in the TACE-surgery group and the surgery group.
Our data has weak points as a retrospective small-sized study. First, since surgery was done 322.7±403.6 days after initial diagnosis of HCC in the TACE-surgery group, HCC with aggressive tumor biology would be excluded during follow up with TACE and enrolled in this study. However, it is also possible that excellent responders to TACE (HCC with favorable tumor biology) were excluded. Second, while the TACE-surgery group and the surgery group shows no significant difference in all baseline characteristics, this similarity might come from small size of this study. For example, a proportion of patients with stage III and IVa HCC in the TACE-surgery group was higher than that in the surgery group (31.2% vs. 15.6%), although the difference did not reach clinical significance. However, subgroup analysis showed overall survival rates were not significantly different between two groups. In addition, whereas the 1-, 2-, and 5-year overall survival rates of the TACE-surgery group were not significantly different from those of the surgery group (78%, 60%, and 26% vs. 97%, 83%, and 45% respectively; P=0.11), statistically significant difference might be demonstrated if large number of patients were included in the analysis.
In conclusion, our results suggest that HCC patients who underwent TACE before resection could have overall survival rates comparable to those without pre-operative therapy, although recurrence rates would be higher in patients with preoperative TACE. However, further large-scaled studies are warranted to clarify the effect of preoperative TACE on the outcome of patients with HCC after surgical resection.
REFERENCES
1. Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer 2001;94:153-156. 11668491.


3. Sugioka A, Tsuzuki T, Kanai T. Postresection prognosis of patients with hepatocellular carcinoma. Surgery 1993;113:612-618. 8389492.

4. Liver Cancer Study Group of Japan. Primary liver cancer in Japan. Clinicopathologic features and results of surgical treatment. Ann Surg 1990;211:277-287. 2155591.


5. Choi TK, Edward CS, Fan ST, Francis PT, Wong J. Results of surgical resection for hepatocellular carcinoma. Hepatogastroenterology 1990;37:172-175. 1692802.

6. Nagorney DM, van Heerden JA, Ilstrup DM, Adson MA. Primary hepatic malignancy: surgical management and determinants of survival. Surgery 1989;106:740-748 discussion 748-749. 2799650.

7. Paquet KJ, Koussouris P, Mercado MA, Kalk JF, Müting D, Rambach W. Limited hepatic resection for selected cirrhotic patients with hepatocellular or cholangiocellular carcinoma: a prospective study. Br J Surg 1991;78:459-462. 1851652.


8. Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology 2004;127(5 Suppl 1):S179-S188. 15508083.


9. Lau WY, Yu SC, Lai EC, Leung TW. Transarterial chemoembolization for hepatocellular carcinoma. J Am Coll Surg 2006;202:155-168. 16377509.


10. Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005;42:1208-1236. 16250051.


11. Yu YQ, Xu DB, Zhou XD, Lu JZ, Tang ZY, Mack P. Experience with liver resection after hepatic arterial chemoembolization for hepatocellular carcinoma. Cancer 1993;71:62-65. 8380123.


12. Fan J, Tang ZY, Yu YQ, Wu ZQ, Ma ZC, Zhou XD, et al. Improved survival with resection after transcatheter arterial chemoembolization (TACE) for unresectable hepatocellular carcinoma. Dig Surg 1998;15:674-678. 9845635.


13. Majno PE, Adam R, Bismuth H, Castaing D, Ariche A, Krissat J, et al. Influence of preoperative transarterial lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis. Ann Surg 1997;226:688-701 discussion 701-703. 9409568.



14. Ochiai T, Sonoyama T, Hironaka T, Yamagishi H. Hepatectomy with chemoembolization for treatment of hepatocellular carcinoma. Hepatogastroenterology 2003;50:750-755. 12828078.

15. Lu CD, Peng SY, Jiang XC, Chiba Y, Tanigawa N. Preoperative transcatheter arterial chemoembolization and prognosis of patients with hepatocellular carcinomas: retrospective analysis of 120 cases. World J Surg 1999;23:293-300. 9933702.


16. Sugo H, Futagawa S, Beppu T, Fukasawa M, Kojima K. Role of preoperative transcatheter arterial chemoembolization for resectable hepatocellular carcinoma: relation between postoperative course and the pattern of tumor recurrence. World J Surg 2003;27:1295-1299. 14574482.


17. Wu CC, Ho YZ, Ho WL, Wu TC, Liu TJ, P'eng FK. Preoperative transcatheter arterial chemoembolization for resectable large hepatocellular carcinoma: a reappraisal. Br J Surg 1995;82:122-126. 7881929.


18. Uchida M, Kohno H, Kubota H, Hayashi T, Yamanoi A, Kimoto T, et al. Role of preoperative transcatheter arterial oily chemoembolization for resectable hepatocellular carcinoma. World J Surg 1996;20:326-331. 8661839.


19. Sasaki A, Iwashita Y, Shibata K, Ohta M, Kitano S, Mori M. Preoperative transcatheter arterial chemoembolization reduces long-term survival rate after hepatic resection for resectable hepatocellular carcinoma. Eur J Surg Oncol 2006;32:773-779. 16797156.


20. Korean Liver Cancer Study Group and National Cancer Center, Korea. Practice guidelines for management of hepatocellular carcinoma 2009. Korean J Hepatol 2009;15:391-423. 19783891.


21. The Korean Liver Cancer Study Group. The General Rules for the Study of Primary Liver Cancer. 2004. 2nd ed. Seoul: Sunmungak; p. 1-56.
22. Nagasue N, Galizia G, Kohno H, Chang YC, Hayashi T, Yamanoi A, et al. Adverse effects of preoperative hepatic artery chemoembolization for resectable hepatocellular carcinoma: a retrospective comparison of 138 liver resections. Surgery 1989;106:81-86. 2545011.


23. Bonfil RD, Bustuoabad OD, Ruggiero RA, Meiss RP, Pasqualini CD. Tumor necrosis can facilitate the appearance of metastases. Clin Exp Metastasis 1988;6:121-129. 3345611.


24. Lee YT, Geer DA. Primary liver cancer: pattern of metastasis. J Surg Oncol 1987;36:26-31. 3041113.


Figure 1
Overall survival rates after surgery in HCC patients with or without preoperative TACE. The 1-, 2-, and 5-year overall survival rates did not differ between the TACE-surgery group and the surgery group (78%, 60%, and 26%, respectively, vs. 97%, 83%, and 45%, respectively; P=0.11).

Figure 2
Recurrence-free survival rates after surgery in HCC patients with or without preoperative TACE. The 1-, 2-, and 5-year recurrence-free survival rates in the TACE-surgery group were 58%, 36%, and 7%, respectively, and were significantly lower than those in the surgery group (77%, 58%, and 32%, respectively; P=0.01).
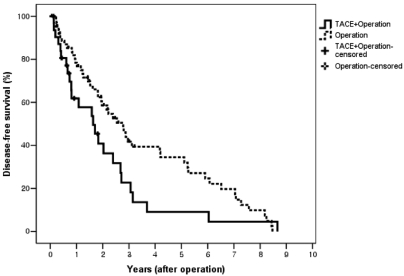
Figure 3
Cumulative recurrence rate after surgery in HCC patients with or without preoperative TACE. The 1-, 2-, and 5-year cumulative recurrence rates in the TACE-surgery group were 48%, 66%, and 77%, respectively, and were significantly higher than those in the surgery group (29%, 46%, and 55%, respectively; P=0.04).
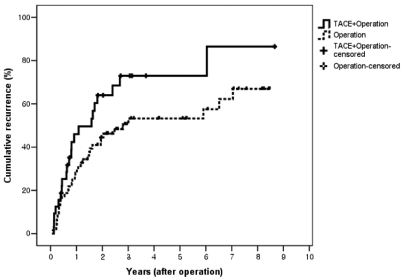
Table 1
Baseline characteristics of patients in the preoperative transarterial chemoembolization (TACE)-surgery and surgery groups





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




