Sarcomatoid cholangiocarcinoma with osteoclast-like giant cells associated with hepatolithiasis: A case report
Article information
INTRODUCTION
Cholangiocarcinoma (CC) is an adenocarcinoma arising from epithelial cells of varying locations within the biliary tree, and is the second most common primary liver tumor, accounting for approximately 10-15% of all hepatobiliary malignancies.1 This disease has a high prevalence in Southeast and Eastern Asia, including Korea; during the period 2003-2005, the age-standardized incidence rates of intrahepatic CC and extrahepatic CC were reported to be 6.9 and 5.2 out of 100,000 in Korea, respectively.2 Sarcomatoid carcinoma is a rare tumor composed of mixed malignant epithelial and mesenchymal cells, and it can occur in various organs including liver. The prevalence of sarcomatoid CC has been reported to be about 4.5% of surgical and autopsied cases.3 Most sarcomatoid CCs have been reported to have sarcomatous component which show spindle cell or pleomorphic cell differentiation.4 Furthermore, sarcomatoid CC with osteoclast-like giant cells, which morphologically resemble those found in giant cell tumors of the bone is a very rare malignant liver tumor which was first reported as osteoclastoma-like giant cell tumor of the liver by Munoz et al. in 1980.5 To the best of our knowledge, sarcomatoid CC with osteoclast-like giant cells are extremely rare and only 5 cases were reported in the literature so far.6 Here we report a case of sarcomatoid CC with osteoclast-like giant cells, which is associated with hepatolithiasis.
CASE SUMMARY
A 67-year-old woman was referred to our hospital for right upper quadrant abdominal pain for 3 weeks. She had no history of alcohol ingestion or smoking, and no remarkable medical history. A computed tomography scan was performed to evaluate the cause of abdominal pain, and revealed distal common bile duct stone or sludge with dilatation of the common bile duct and bilateral intrahepatic ducts and a 6 cm-sized heterogeneous lobulated mass in the left lateral segment of the liver, suggestive of intrahepatic CC with multiple left intrahepatic duct stones (Fig. 1A, B). There was no definite evidence of hepatic artery or portal vein invasion or intrahepatic metastasis. Initial laboratory findings showed normal liver function tests: aspartate aminotransferase 16 IU/L, alanine aminotransferase 22 IU/L, total bilirubin 0.4 mg/dL, direct bilirubin 0.1 mg/dL and elevated gamma-glutamyl transpeptidase 97 IU/L and alkaline phosphatase 151 IU/L. Tumor marker tests revealed elevated carbohydrate antigen 19-9 (1598.0 U/mL) and carcinoembryonic antigen (109.60 ng/mL) levels. Testing for hepatitis B and hepatitis C virus were negative. Under the impression of mass-forming intrahepatic CC, she underwent a left lobectomy of the liver.
PATHOLOGIC FINDINGS
On gross examination, the cut surface revealed a single, firm, whitish to tan mass (4.5×4.0 cm) with periductal infiltration around the dilated left intrahepatic duct. The duct lumen was distended to about 2 cm in diameter with 3 cm sized black stone impaction (Fig. 2). Microscopically, the tumor showed two distinct components; carcinomatous component and sarcomatous component (Fig. 3A, 4A). In the carcinomatous component, the malignant cells formed irregular papillae or fused glands in a prominent fibrous stroma, consistent with features of a typical well-to-moderately differentiated adenocarcinoma. The columnar-to-cuboidal epithelial neoplastic cells had atypical nuclei with some prominent nucleoli, slightly eosinophilic and granular cytoplasm, and some mitosis (Fig. 3B). In the sarcomatous component, there were pleomorphic cells and atypical spindle cells with vesicular nuclei and prominent nucleoli (Fig. 3C). The two components were intermingled. In addition, there were many scattered osteoclast-like giant cells: large multinucleated giant cells containing 10-25 nuclei and about 150-200 µm in diameter (Fig. 4F). Perineural invasion and microvessel invasion were seen. In surrounding liver, hepatolithiasis with chronic proliferative cholangitis and microabscess formation was noted (Fig. 3D). The immunohistochemical staining results also demonstrated two distinct patterns. The carcinomatous portion was positive for cytokeratin 19 (Fig. 4B) and negative for vimentin. The sarcomatous portion was positive for vimentin (Fig. 4E) and negative for cytokeratin 19. Both were negative for hepatocyte antigen and alpha-fetoprotein. The osteoclast-like giant cells were positive for CD68 (PG-M1) (Fig. 4F). The immunohistochemical staining with EMT-related markers, E-cadherin and uPAR was conducted. E-cadherin showed total loss in the sarcomatous portion and no loss in the carcinomatous portion (Fig. 4C). uPAR was positive in the sarcomatous portion and peritumoral stroma but negative in the carcinomatous portion (Fig. 4D).

Gross finding. The representative cut surface reveals a single, firm, whitish to tan mass (4.5×4.0 cm) with periductal infiltration around the dilated left intrahepatic duct. The lumen of bile duct is distended up to 2 cm in diameter and impacted with pigment stone.
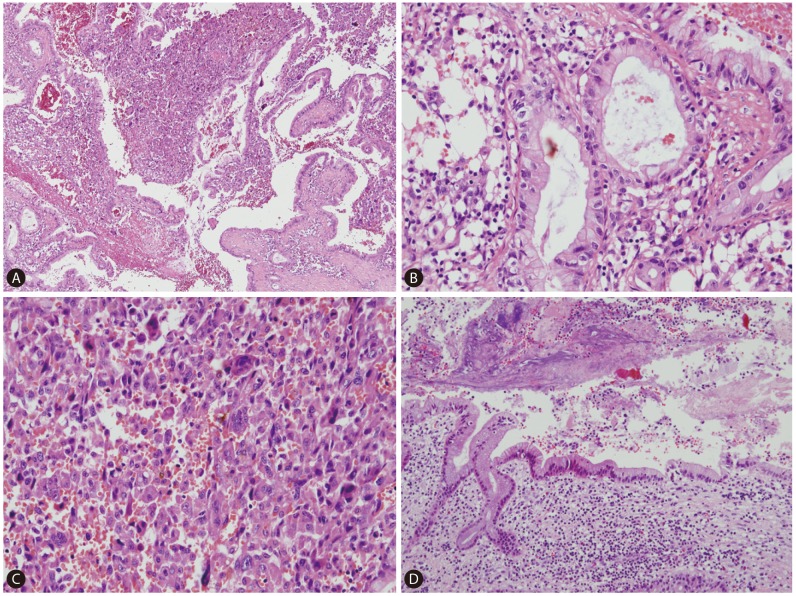
Microscopic finding. Carcinomatous component and sarcomatous component are intermingled (A. H-E, ×40). Carcinomatous component shows the features of typical well-to-moderately differentiated adenocarcinoma (B. H-E, ×200). Sarcomatous component is consisted with pleomorphic cells and atypical spindle cells (C. H-E, ×200). In surrounding liver, hepatolithiasis with chronic proliferative cholangitis and microabscess formation are noted (D. H-E, ×100).

Immunohistochemical finding. Microscopic feature of sarcomatoid cholangiocarcinoma shows intermingled carcinomatous and sarcomatous component (A. H-E, ×100). Carcinomatous component shows strong expression of cytokeratin 19, but sarcomatous component shows no expression of cytokeratin 19 (B. CK19, ×200). E-cadherin shows total loss in the sarcomatous portion and no loss in the carcinomatous portion (C. E-cadherin, ×200). uPAR is positive in the sarcomatous portion but negative in the carcinomatous portion (D. uPAR, ×200). Sarcomatous component shows diffuse and strong expression of vimentin (E. vimentin, ×200). Many scattered osteoclast-like giant cells shows positive expression of CD68 (PG-M1) (F. H-E and CD68 (PG-M1), ×400).
DISCUSSION
Sarcomatoid CC is an extremely rare liver primary tumor, and its pathogenesis still remains unclear. Sarcomatous change may be explained by epithelial-mesenchymal transition (EMT), which refers to the biologic process of epithelial phenotype changing to mesenchymal phenotype by undergoing multiple biochemical changes including enhanced migratory capacity, invasiveness, elevated resistance to apoptosis, and increased production of extracellular matrix components. A hallmark of EMT is the loss of epithelial characteristics such as a decrease in the expression of the cell adhesion molecule E-cadherin and acquisition of a mesenchymal phenotype accompanied by increased expression of vimentin. Transforming growth factor β as well as transcription factors such as Twist, Snail, Slug, Sip1, ZEB1 and ZEB2 have a regulatory role in EMT.7 Also, increased uPAR expression has been implicated in the promotion of EMT in various cancers.8 Previous report about 27 cases of sarcomatoid carcinoma originating from various organs, mostly from gastrointestinal tract which included 4 cases of CCs demonstrated that most sarcomatoid carcinomas express EMT-related markers such as Twist1, Snail1, Slug, Sip1 and ZEB1.9 Presenting case showed a positive expression for EMT-related markers: vimentin, uPAR, and loss of E-cadherin, suggesting of EMT.
Another sarcomatoid carcinoma primarily occurring in the liver is sarcomatoid hepatocellular carcinoma (HCC), and 73 cases of sarcomatoid HCC have been reported in the English literature in the past 20 years.10 Preoperative anticancer treatment such as transcatheter arterial chemoembolization, radiofrequency ablation or percutaneous ethanol injection has been reported to be associated with sarcomatous change in HCC.11 Sarcomatoid HCC without previous therapies was also reported, although there are only a few reports of such cases.12 The present patient with sarcomatoid CC had no history of preoperative treatment, therefore, preoperative treatment is not considered to be necessary for the sarcomatoid change.
Interestingly, this case showed osteoclast-like giant cells in addition to spindle cells of sarcomatous component. Tumors that contain osteoclast-like giant cells have been reported in several sites, which include the pancreas, lung, thyroid gland, stomach, breast, uterus, kidney, soft tissue, salivary glands,13 and liver.14 Osteoclast-like giant cells which closely resemble those of giant cell tumors of bone may be derived from bone marrow monocytes as they express the monocytic/histiocytic marker, CD6815 and are negative for the epithelial markers. In this case, they were positive for CD68, and were negative for the epithelial marker, cytokeratin 19. These findings support that osteoclast-like giant cells are not epithelial origin but monocytic/histiocytic origin.
This presenting case showed chronic cholangitis with hepatolithiasis in the non-neoplastic liver. Clinically, hepatolithiasis is considered as a main cause of CC in Korea16 and approximately 10% of patients with hepatolithiasis develop CC.17 Repeated episodes of cholangitis due to hepatolithiasis are considered to induce proliferative epithelial changes and accelerate tumorigenesis.18
The prognosis of CC is generally unfavorable because of frequent metastasis, low resectability rate and high incidence of recurrence even after curative resection. The median survival is 27 months, and 5-year survival was 31%.19 The outcome of sarcomatoid CC appears to be even worse than conventional CC without sarcomatous change.20 Previous reports have demonstrated that these tumors are consistently very aggressive because of intrahepatic metastasis and frequent widespread metastasis of sarcomatoid cells.3 Therefore, early detection and complete surgical resection might be the only chance to improve the patient's outcome.21 The present case had no intrahepatic metastasis or extrahepatic metastasis at the time of operation, and there was no evidence of cancer recurrence at 6 months after operation.
Notes
Conflicts of Interest: The authors have no conflicts to disclose.
Abbreviations
CC
cholangiocarcinoma
EMT
epithelial-mesenchymal transition
HCC
hepatocellular carcinoma
