| Clin Mol Hepatol > Volume 21(2); 2015 > Article |
Tissue confirmation is a gold standard for diagnosing most malignant tumors. However, widely accepted guidelines for management of hepatocellular carcinoma (HCC) enable making a diagnosis of HCC based on not only biopsy but also imaging techniques,1,2 because biopsy carries the potential risks of mistargeting, sampling error, and complications including bleeding and needle track seeding. The diagnostic accuracy of dynamic imaging technique showing arterial hypervascularity and washout of contrast in the venous-delayed phases has been demonstrated by several studies.3,4 In addition, recent advances in MR techniques and new hepatocyte-specific contrast agents improved diagnostic accuracy of imaging technique.
Although diagnostic accuracy of imaging technique for HCC has been improved, misdiagnosis is encountered not uncommonly in real clinical practice. Both false positive diagnosis and false negative diagnosis cause serious problem of patient-doctor relationship. To reduce the rate of misdiagnosis, doctors should be familiar with the clinical manifestation and the imaging findings of those cases. We here report three false positive cases of benign hepatic nodules mimicking HCC.
A 59-year-old male patient with hepatitis B virus cirrhosis, who was under surveillance for HCC, showed a mass on a computed tomography (CT) scan. The mass was not seen on the previous CT scan which was performed 15 months ago. Arterial phase images showed a 1-cm hypervascular mass in the subcapsular area of segment V/VIII of the liver (Fig. 1). However, the mass did not show washout of contrast on delayed phase images. Gadoxetic acid-enhance Liver magnetic resonance imaging (MRI) was performed three months later for further evaluation. There was no significant interval change in size. The mass showed low signal intensity (SI) on T1-weighted images (T1WI), high SI on T2-weighted images (T2WI), hypervascularity on arterial phase, washout of contrast on portal and delayed phases, and low SI on hepatobiliary phase. The mass demonstrated high SI on diffusion-weighted images (DWI) and low apparent diffusion coefficient (ADC) value. Liver function tests showed normal range of aspartate aminotransferase (AST) and alanine aminotransferase (ALT). The serum tumor marker, ╬▒-fetoprotein (AFP) was also normal range. Our impression was HCC. He underwent tumorectomy. However, the histologic diagnosis was intrahepatic bile duct adenoma on a background of hepatitis B virus cirrhosis.
A 30-year-old female patient was referred to our hospital for evaluation of a hepatic tumor which was incidentally detected during ultrasound (US) screening at a local clinic. The patient had no symptom or history of viral hepatitis or excessive alcohol intake. All liver function tests and AFP were normal range. On outside US, there was a 1.6 cm hyperechoic mass in segment IV/I of the liver (Fig. 2). On contrast enhanced CT scan, the mass showed low density on precontrast images, hypervascularity on arterial phase, and washout of contrast on portal and delayed phases. For further evaluation, gadoxetic acid-enhanced liver MRI was performed. On T1-weighted in-phase and out-of-phase images, the mass contained focal fat tissue. On arterial phase, early draining vein was not seen but the left hepatic vein showed early enhancement. The mass showed low SI on T1WI, high SI on T2WI, high SI on DWI, and low SI on hepatobiliary phase (Fig. 2). Our first impression was fat-containing HCC. However, she did not have any risk factors for HCC, and underwent percutaneous needle biopsy. The histologic diagnosis was hepatic angiomyolipoma. Conservative management with close follow-up was decided because she did not have any symptoms and the size of tumor was relatively small. However, the size increased to 2.5 cm on follow-up MRI performed 9 months later. Finally, she underwent left hemihepatectomy and caudate lobectomy, and angiomyolipoma was confirmed.
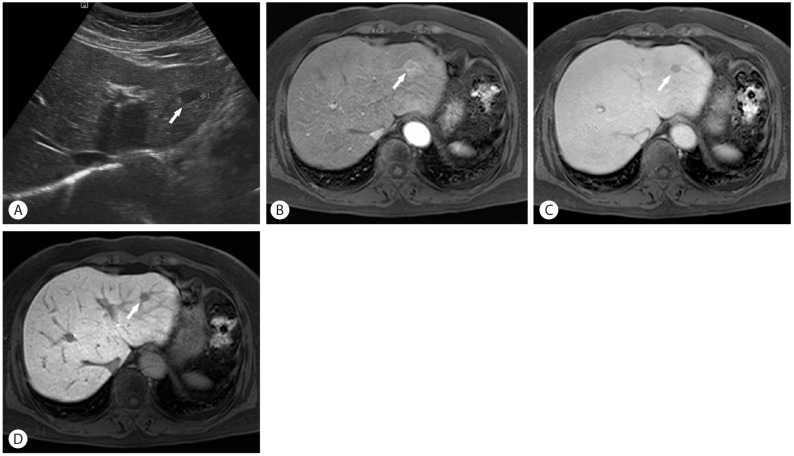
A 66-year-old female patient was referred to our hospital for evaluation of a hepatic tumor which was incidentally detected during US screening at a local clinic. She had a medical history of chronic renal failure. The patient had no history of viral hepatitis or excessive alcohol intake. All liver function tests and AFP were normal range. Outside US showed a 1.2 cm sized hypoechoic mass in left lateral segment of the liver (Fig. 3). It was a low density mass on precontrast CT images. On the contrast-enhanced dynamic CT images, the mass revealed subtle enhancement on arterial phase and washout of contrast on delayed phase. On gadoxetic acid-enhance MRI, the mass showed low SI on T1WI, high SI on T2WI, and high SI on DWI. On arterial phase images, the peripheral portion showed more intense enhancement than the central portion. The peripheral intense-enhancing portion showed iso SI on hepatobiliary phase, but the central less-enhancing portion showed low SI on hepatobiliary phase. Considering this imaging finding, the peripheral intense enhancement could be considered as perinodular enhancement not enhancement of tumor itself. The perinodular enhancement was sustained until the delayed phase. Our initial impression was HCC or other hypervascular malignant tumors. We tried to perform percutaneous needle biopsy, but it was too risky to perform it due to surrounding vessels. Finally, she underwent laparoscopic lateral sectionectomy. The histologic diagnosis was pseudolymphoma.
Bile duct adenoma is a rare benign tumor arising from the epithelium of bile ducts. As the patients usually do not present with clinical symptoms, this tumor is detected incidentally. Its incidence was reported to about 1.3% of primary hepatic tumors.5 The mean age at diagnosis is 55 years without gender difference in the incidence of this disease.6
Histologically, bile duct adenoma is characterized by inflammation and fibrosis involving a proliferation of bile ductules, which are thought to be reactive processes in response to focal injuries and may be associated with chronic liver disease. As the disease progresses, fibrous tissues increases. Most cases were small (<2 cm) and typically found on the surface of the liver. It is a usually solitary nodule, but may occur as multiple nodules.
Although bile duct adenoma is defined as a benign tumor, there was a case report suggesting the possibility of cholangiocarcinoma associated with bile duct adenoma.7 However, so far there is no report about tumor recurrence in the literature and patients have a good prognosis.
There have been several reports on the imaging findings of bile duct adenoma. According to the previous reports, many of bile duct adenomas showed hypervascularity on arterial phase. However, SI on T2-weighted images and washout of contrast on delayed phase were variable probably according to the amount of fibrous stromal component. A previous case report suggested that high ADC value could be used an important tool to reveal the benignity of bile duct adenoma,8 but ADC value was low in our case. It is natural that bile duct adenoma shows low SI on hepatobiliary phase because it originated from bile duct epithelium not hepatocyte.
Hepatic angiomyolipoma is a rare benign mesenchymal tumor. There is marked female predominance. It composed of proliferating blood vessels, smooth muscle cells, and adipose cells. Definite diagnosis is made by identification of these three components and HMB-45 positive staining.
In the past, this tumor has been considered as an entirely benign and slow-growing lesion without possibility of malignant transformation. However, the cases showing aggressive changes such as growth in size, recurrence after surgical resection, metastasis, and invasive growth pattern have been reported. Based on these reports, a study recommended that all patients with symptoms or tumor larger than 5 cm receive surgical resection for hepatic angiomyolipoma.9
Typical imaging findings in imaging studies of hepatic angiomyolipoma are heterogenous hyperechoic mass in US, presence of fat component on CT or MRI and arterial hypervascularity. However, preoperative diagnostic accuracy for hepatic angiomyolipoma has not been high because hepatic angiomyolipoma shows various patterns in imaging studies according to the relative proportions of vessels, muscles, and fatty tissue. Most common impression of misdiagnosed cases was HCC because HCC also is a hypervascular tumor and can contain a considerable fat content. According to the previous reports, there are several points which may be useful in discriminating between angiomyolipoma and HCC: (A) presence of early draining vein, (B) absence of tumor capsule, and (C) peripheral decreasing enhancing rim.10,11
Hepatic pseudolymphoma, also known as reactive lymphoid hyperplasia is a rare benign nodular lesion. The average age of the patients was 56.7 years with female predominance. Most of the tumors were no more than 2 cm and single. Although the precise etiology is still unknown, the relatively high prevalence of autoimmune disorders suggests a possible involvement of autoimmunity to hepatic pseudolymphoma.
Histologically, it consists of tumorous infiltrates of mature lymphocytes with multiple lymphoid follicles or clusters of epithelioid histiocytes. Lymphocytes characteristically extend into nearby portal tracts.12
Pseudolymphoma tends to show low density on precontrast CT images, slight early enhancement in the arterial phase, and low to isodensity in the delayed phase. On gadoxetic acid-enhanced MRI, the nodules reveal low SI on T1WI, high SI on T2WI, high SI on DWI, and low SI on the hepatobiliary phase. However, these imaging findings are not specific for pseudolymphoma. Therefore, most reported cases have been disdiagnosed as HCC or metastatic carcinoma. Meanwhile, a recent study reported that hepatic pseudolymphoma showed unique enhancement pattern, named perinodular enhancement on dynamic contrast-enhanced CT or MRI. The perinodular enhancement reflects increased arterial supply in perinodular hepatic parenchyma caused by portal venular stenosis and/or disappearance due to marked lymphoid cell infiltration in perinodular portal tracts.13 This finding may be useful in the differentiation from HCC or other tumors.
In conclusion, benign lesions such as bile duct adenoma, angiomyolipoma, and pseudolymphoma can mimic HCC. However, there are several specific clinical manifestations and imaging findings that may be useful in the differentiation between these lesions and HCC. Therefore, doctors should be familiar with them to reduce misdiagnosis.
Although diagnostic accuracy of imaging techniques for HCC has been improved through recent advances in MR techniques and new hepatocyte-specific contrast agents, misdiagnosis is encountered not uncommonly in real clinical practice. To reduce the rate of misdiagnosis, doctors should be familiar with the clinical manifestation and the imaging findings of false positive and false negative cases. We here report three cases with benign hepatic nodules mimicking HCC: bile duct adenoma, angiomyolipoma and pseudolymphoma.
Abbreviations
HCC
hepatocellular carcinoma
CT
computed tomography
MRI
magnetic resonance imaging
SI
signal intensity
T1WI
T1-weighted images
T2WI
T2-weighted images
DWI
diffusion-weighted images
ADC
apparent diffusion coefficient
AST
aspartate aminotransferase
ALT
alanine aminotransferase
AFP
╬▒-fetoprotein
US
ultrasound
REFERENCES
1. Bruix J, Sherman M. American Association for the Study of Liver D. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-1022. 21374666.



2. European Association For The Study Of The Liver. European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-943. 22424438.


3. Forner A, Vilana R, Ayuso C, Bianchi L, Sole M, Ayuso JR, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 2008;47:97-104. 18069697.


4. Sangiovanni A, Manini MA, Iavarone M, Romeo R, Forzenigo LV, Fraquelli M, et al. The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut 2010;59:638-644. 19951909.


5. Edmondson HA. Tumors of the liver and intrahepatic bile ducts. Atlas of tumor pathology. Washington DC: Armed Forces Institute of Pathology; 1958. p. 19-29.
6. Allaire GS, Rabin L, Ishak KG, Sesterhenn IA. Bile duct adenoma. A study of 152 cases. Am J Surg Pathol 1988;12:708-715. 3046396.


7. Hasebe T, Sakamoto M, Mukai K, Kawano N, Konishi M, Ryu M, et al. Cholangiocarcinoma arising in bile duct adenoma with focal area of bile duct hamartoma. Virchows Arch 1995;426:209-213. 7757293.


8. An C, Park S, Choi YJ. Diffusion-weighted MRI in intrahepatic bile duct adenoma arising from the cirrhotic liver. Korean J Radiol 2013;14:769-775. 24043970.



9. Yang CY, Ho MC, Jeng YM, Hu RH, Wu YM, Lee PH. Management of hepatic angiomyolipoma. J Gastrointest Surg 2007;11:452-457. 17436129.



10. Cai PQ, Wu YP, Xie CM, Zhang WD, Han R, Wu PH. Hepatic angiomyolipoma: CT and MR imaging findings with clinical-pathologic comparison. Abdom Imaging 2013;38:482-489. 22996326.


11. Jeon TY, Kim SH, Lim HK, Lee WJ. Assessment of triple-phase CT findings for the differentiation of fat-deficient hepatic angiomyolipoma from hepatocellular carcinoma in non-cirrhotic liver. Eur J Radiol 2010;73:601-606. 19200676.


Figure┬Ā1
A 59-year-old male patient with bile duct adenoma (arrows) in segment V/VIII of the liver. The nodule shows arterial hypervascularity (A) but does not show washout of contrast on delayed phase of CT (B). On MRI, it shows arterial hypervascularity (C) and washout of contrast on delayed phase images (D). It shows high SI on T2WI (E). It reveals diffusion restriction (F). CT, computed tomography; MRI, magnetic resonance imaging; SI, signal intensity; T2WI, T2-weighted images.

Figure┬Ā2
A 30-year-old female patient with hepatic angiomyolipoma (arrows) in segment IV/I of the liver. There is a 1.6 cm sized hyperechoic mass in segment IV/I (A). On T1-weighed in-phase (B) and out-of-phase (C) images, there is a focal fatty area within the tumor (white arrow head). On arterial phase of MRI (D), the early draining vein is not definite but the left hepatic vein shows early enhancement (black arrow head). It shows low SI on hepatobiliary phase (E) and diffusion restriction (F). MRI, magnetic resonance imaging; SI, signal intensity.

Figure┬Ā3
A 66-year-old female patient with hepatic pseudolymphoma (arrows) in left lateral segment of the liver. There is a 1.2 cm sized hypoechoic mass on US (A). There is a perinodular enhancement on arterial phase of MRI (B) and the perinodular enhancement is sustained until delayed phase (C). It shows low SI on hepatobiliary phase (D). MRI, magnetic resonance imaging; SI, signal intensity.
- TOOLS
-
METRICS

- Related articles
-
Surveillance of hepatocellular carcinoma: is only ultrasound enough?2017 September;23(3)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



