Cholangiolocellular carcinoma with satellite nodules showing intermediate differentiation
Article information
INTRODUCTION
Cholangiolocellular carcinoma, a rare type of primary malignant tumor of liver, is thought to originate from cholangioles or canals of Hering, where hepatic progenitor or stem cells are located,12 and has characteristic histologic features of tubular, cord-like and 'antler-like' pattern with marked fibrous stroma.3 Cholangiolocellular carcinoma was previously classified as one type of intrahepatic cholangiocarcinoma. But now, on the basis of the concept that combined hepatocellular-cholangiocarcinoma is derived from hepatic progenitor cells, cholangiolocellular carcinoma is classified as combined hepatocellular-cholangiocarcinoma with stem-cell features in the latest version of the World Health Organization (WHO) classification of the digestive system 2010.3 Because of its limited number of cases, its biologic behavior or clinical outcomes have not been fully understood.
CASE SUMMARY
A 62-year-old male presented with abdominal pain and dyspnea. He was a chronic alcoholic and had no positive markers for hepatitis virus. On physical examination, he showed epigastric tenderness. Initial serum aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase levels were 48 IU/L, 19 IU/L and 167 IU/L, respectively. Serum alpha-fetoprotein, carcinoembryonic antigen and carbohydrate antigen 19-9 levels were 4.9 ng/mL, 2.2 ng/mL and 12.1 U/mL, respectively. Abdominal computed tomography and magnetic resonance imaging showed a 5 cm-sized peripheral enhancing lesion with centripetal enhancement pattern, and multiple arterial enhancing nodules in right hepatic lobe. Under the impression of intrahepatic malignant tumor, he underwent a right lobectomy of liver.
PATHOLOGICAL FINDINGS
On gross examination, liver surface showed multiple protruding nodules. On serial section of the liver, there was a 5 cm-sized whitish firm and fibrotic mass and more than 10 multiple small satellite nodules with same consistency and color of the largest mass (Fig. 1). Microscopically, the 5 cm-sized largest mass showed tumor cells arranged in tubular, cord-like and anastomosing histologic pattern, so called "antler-like" pattern with mild nuclear atypia in the marked fibrous stroma of which the typical findings of cholangiolocellular carcinoma (Fig. 2). Most of other satellite nodules showed same histologic findings. But, there were two satellite nodules which revealed different histologic findings from other nodules. Tumor cells of these nodules had more abundant cytoplasm than other nodules, showed trabecular or cluster like structural pattern rather than tubular or antler-like pattern, and had scant intervening fibrous stroma. These histologic findings were intermediate differentiation between hepatocellular carcinoma and cholangiocarcinoma. On immunohistochemical stainings, the cells of typical cholangiolocellular carcinoma nodules were positive for keratin 19, but the tumor cells showing intermediate differentiation were negative for keratin 19. Both type cells were negative for hepatocyte paraffin 1 (HepPar-1) which is relatively specific marker for hepatocellular carcinomas and positive for epithelial cell adhesion molecule (EpCAM) which is used as stem/progenitor cell marker (Fig. 3). Through these findings, the diagnosis of combined hepatocellular-cholangiocarcinoma with stem-cell features, cholangiolocellular type could be made.
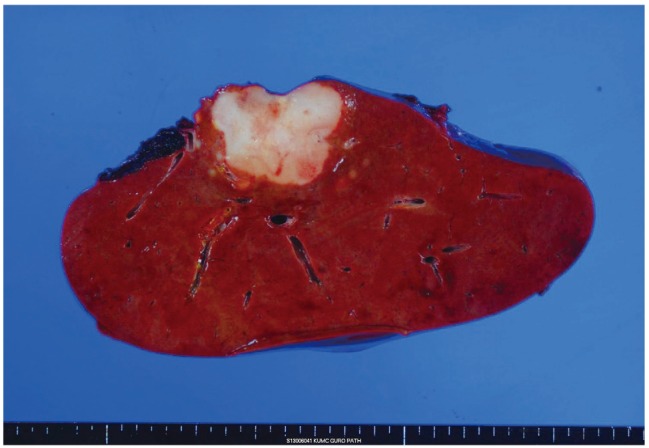
Macroscopic picture of the right lobe of the liver. 5 cm-sized whitish firm and fibrotic mass with multiple small satellite nodules.
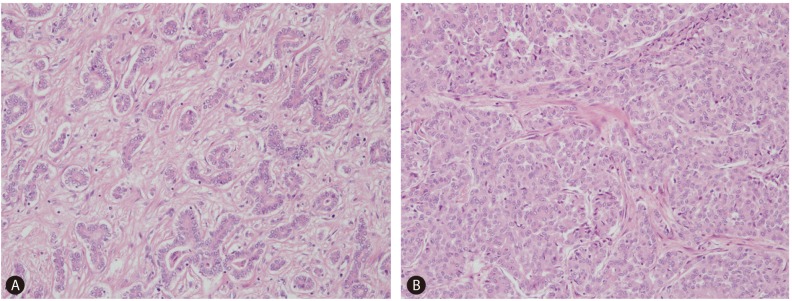
Histologic features of combined hepatocellular-cholangiocarcinoma. (A) Typical cholangiolocellular carcinoma component with tumor cells arranged in tubular and "antler-like" pattern in a markedly fibrous stroma. (B) Satellite nodule showing intermediate differentiation. Tumor cells show more abundant cytoplasm, structural pattern of trabeculae or nest, and scant fibrous stroma (Hematoxylin and eosin, ×200).
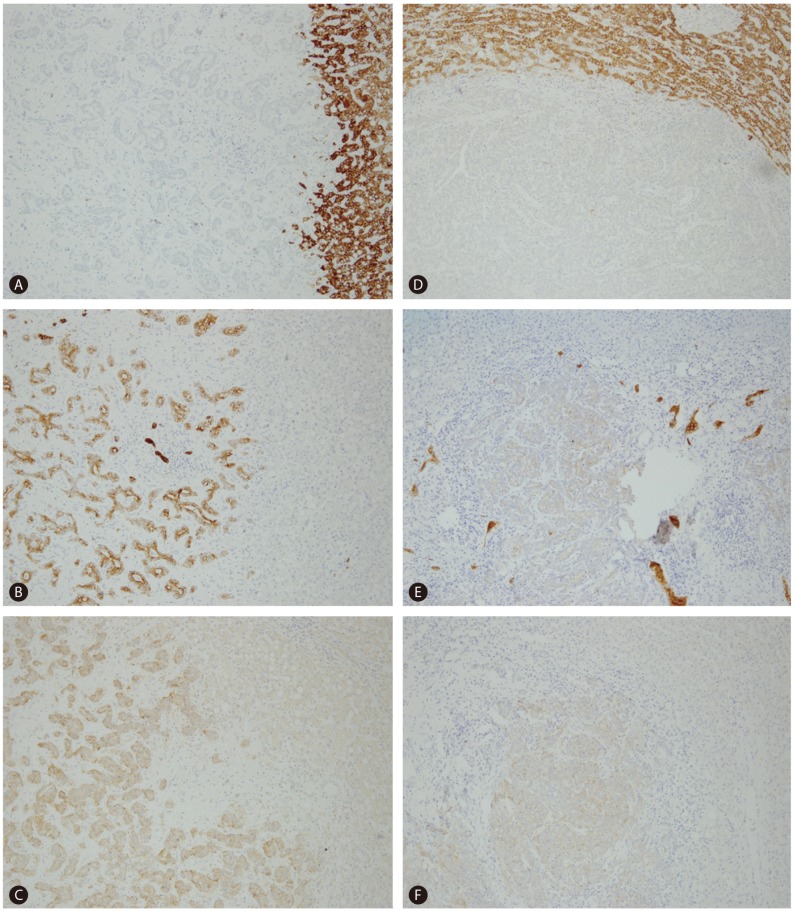
Immunohistochemical findings of typical cholangiolocellular carcinoma component (left column) and intermediate differentiation component (right column). Typical cholangiolocellular carcinoma component shows negativity for HepPar-1 (A) and positive reactivity for keratin 19 (B) and EpCAM (C). Intermediate differentiation component shows negative reactivity for HepPar-1 (D) and keratin 19 (E), and weak positive reactivity for Ep-CAM (F). EpCAM, epithelial cell adhesion molecule; HepPar-1, hepatocyte paraffin 1.
DISCUSSION
Combined hepatocellular-cholangiocarcinoma, a rare primary malignant liver tumor, is composed of unequivocal, intimately mixed components of both hepatocellular carcinoma and cholangiocarcinoma.3 Classical type of combined hepatocellular-cholangiocarcinoma contains both typical hepatocellular carcinoma and typical cholangiocarcinoma area.
Combined hepatocellular-cholangiocarcinoma with stem cell feature is newly adopted in the latest WHO classification of the digestive system 2010. This category includes typical subtype, intermediate-cell subtype and cholangiolocellular subtype.3 Typical subtype is characterized by mature appearing hepatocytes with peripheral cluster of small cells showing positive immunoreactivity for stem/progenitor cell markers.3 Intermediate-cell subtype consists of tumor cells with features intermediate between hepatocytes and cholangiocytes characterized by small and oval-shaped tumor cells with scant cytoplasm and arranged in solid nests and strands within fibrotic stroma with marked desmoplasia.34 Cholangiolocellular type is characterized by tubular, cord-like, anastomosing pattern, the so-called "antler-like" pattern, mild nuclear pleomorphism, and marked fibrous stroma.35
This case showed multiple masses with mainly typical histologic findings of cholangiolocellular carcinoma with tumor cells arranged in tubular, cord-like and "antler-like" pattern in the marked fibrous stroma, and small portion of stem cell-like intermediate differentiation. The immunohistochemical stain for keratin 19 showed positive reactivity in typical cholangiolocellular carcinoma component but negative reactivity in intermediate component. Both areas showed positive reactivity for EpCAM which is a marker for stem/progenitor cells.
Cholangiolocellular carcinoma, now cholangiolocellular type of combined hepatocellular-cholangiocarcinoma with stem cell features, was traditionally classified as a special type of intrahepatic cholangiocarcinoma. In the latest classification of WHO digestive system, cholangiolocellular carcinoma was reclassified into combined hepatocellular-cholangiocarcinoma with stem cell features, because this type of tumor shows histologic and immunohistochemical differentiation of hepatic progenitor/stem cells and often shows focal differentiation of hepatocellular carcinoma or intermediate differentiation between hepatocellular carcinoma and cholangiocarcinoma.12 However, its clinical outcome has not been clarified because of its limited number of cases. There are some reports not only that cholangiolocellular carcinoma has poor outcome as much as intrahepatic cholangiocarcinoma but also that cholangiolocellular carcinoma has favorable long-term survival.67 There are two studies for clinicopathological analysis of combined hepatocellular-cholangiocarcinoma according to the latest WHO classification.89 But these studies also had limited number of cases (less than 100 patients). So it is required more cases and studies for evaluation of prognosis and treatment strategy in cholangiolocellular carcinoma patients.
Notes
Conflicts of Interest: The authors have no conflicts to disclose.
Abbreviations
EpCAM
epithelial cell adhesion molecule
HepPar-1
hepatocyte paraffin 1
WHO
World Health Organization