| Clin Mol Hepatol > Volume 20(1); 2014 > Article |
ABSTRACT
Background/Aims
To determine the value of fusion imaging with contrast-enhanced ultrasonography (CEUS) and computed tomography (CT)/magnetic resonance (MR) images for percutaneous radiofrequency ablation (RFA) of very-early-stage hepatocellular carcinomas (HCCs) that are inconspicuous on fusion imaging with B-mode ultrasound (US) and CT/MR images.
Methods
This retrospective study was approved by our institutional review board and the requirement for informed consent was waived. Fusion imaging with CEUS using Sonazoid contrast agent and CT/MR imaging was performed on HCCs (<2 cm) that were inconspicuous on fusion imaging with B-mode US. We evaluated the number of cases that became conspicuous on fusion imaging with CEUS. Percutaneous RFA was performed under the guidance of fusion imaging with CEUS. Technical success and major complication rates were assessed.
Results
In total, 30 patients with 30 HCCs (mean, 1.2 cm; range, 0.6-1.7 cm) were included, among which 25 (83.3%) became conspicuous on fusion imaging with CEUS at the time of the planning US and/or RFA procedure. Of those 25 HCCs, RFA was considered feasible for 23 (92.0%), which were thus treated. The technical success and major complication rates were 91.3% (21/23) and 4.3% (1/23), respectively.
Fusion imaging of B-mode ultrasonography (US) with liver CT/MR images has been increasingly used for guiding percutaneous radiofrequency ablation (RFA) of small hepatocellular carcinomas (HCCs).1,2 Fused CT/MR images that move synchronously with US transducer enable the operator to correlate real-time B-mode US images with fused CT/MR images and therefore help localize small HCCs inconspicuous on B-mode US. However, accurate localization of true HCC can be still challenging even on fusion imaging when the index tumors are very small or when there are many cirrhosis-related nodules in the surrounding liver.3
Like fusion imaging, contrast-enhanced ultrasonography (CEUS) is also useful for guiding percutaneous RFA of HCCs.4,5,6,7 Among various contrast agents, Sonazoid (perflubutane microbubbles; GE Healthcare, Oslo, Norway), which offers both vascular and post-vascular phases, is known to be very effective in localizing small HCCs inconspicuous on B-mode US.8,9,10 The post-vascular phase, also called the Kupffer phase, is helpful for accurate insertion of RF electrode into the index tumor which is generally seen as a low echogenic defect in the background of well enhancing normal parenchyma. It can be effective for more than 60 minutes, providing a relatively wide temporal window. However, not all small HCCs, especially well-differentiated HCCs, are identifiable on CEUS.4,8
Therefore, when the two techniques used alone are not satisfactory for localizing small HCCs inconspicuous on B-mode US, we believe that two novel techniques coupled simultaneously can be help localize these small inconspicuous HCCs. However, we are unaware of prior data dealing with this issue. In this retrospective study, our aim was to evaluate the value of fusion imaging with CEUS and CT/MR images in performing percutaneous RFA of very early-stage HCCs (<2 cm) inconspicuous on fusion imaging with B-mode US and CT/MR images.
This retrospective study was approved by our institutional review board and informed consent was waived. Inclusion criteria were as follows: (a) Planning US using fusion imaging for percutaneous RFA of HCCs performed between September 2012 and September 2013 in our institution (Samsung Medical Center); (b) Patients with single treatment naïve HCC <2 cm in maximum diameter on CT/MR images (very early-stage in the Barcelona Clinic Liver Cancer staging); (c) Patients with HCC inconspicuous on fusion imaging of B-mode US and CT/MR images; (d) Additional CEUS using Sonazoid during percutaneous RFA; (e) Child-Pugh class A or B; (f) No evidence of vascular invasion or extrahepatic metastases; and (g) Absence of severe coagulopathy (i.e., prothrombin activity <40% or platelet count <40,000/mL). The exclusion criteria were as follows: (a) HCCs located in the sonographically blind area (e.g., hepatic dome); (b) HCCs conspicuous enough on fusion imaging, thus considered feasible for percutaneous RFA; (c) HCCs conspicuous on fusion imaging, but RFA determined to be infeasible for reasons other than conspicuity; and (d) Conventional CEUS mode with the application of CEUS image and B-mode US image side-by-side.
HCC was diagnosed when it showed hyperenhancement in the arterial phase followed by washout in the portal/delayed phase on either dynamic CT or MRI according to the 2010 Asian Pacific Association for the Study of the Liver (APASL) guidelines,11 in which HCC can be diagnosed by dynamic contrast enhanced imaging techniques regardless of tumor size and serum alpha-fetoprotein level. Histopathologic confirmation by percutaneous biopsy was not performed in any patient. The size of the tumor was defined as the maximum diameter on CT or MR images.
Contrast-enhanced three phase liver CT scans were performed using a 64-detector CT scanner (LightSpeed VCT, GE Healthcare, Milwaukee, Wis or Aquilion 64, Toshiba Medical, Ottawara, Japan). Gadoxetic acid (Gd-EOB-DTPA)-enhanced liver MR images were obtained using a 3.0-T MR system (Intera Achieva 3.0-T, Philips Healthcare, Best, the Netherlands) equipped with a 32-channel phased-array coil.
All planning US were conducted to evaluate the feasibility of percutaneous RFA with LOGIQ E9 (GE Healthcare, Waukesha, Wisconsin) equipped with a fusion imaging (Volume Navigation; GE Healthcare, Waukesha, Wisconsin) using a 1-5 MHz convex probe. This was performed by one of the three board-certified abdominal radiologists (M.W.L., H.R., H.K.L.) who each had experience of more than 800 RFA procedures (including more than 100 cases of fusion imaging-guided RFA) at the beginning of this study. Image fusion between B-mode US and CT/MR images was similar to the method of previous studies.3,12 After image fusion, CT or MR images on which the index tumor and anatomic landmarks were most clearly depicted was synchronously displayed side-by-side with the B-mode US images. The radiologist who performed planning US graded conspicuity of the index tumor based on a scoring system according to the degree of confidence using a 4-point scale: score 1, the echogenicity of the index tumor is definitely different from that of surrounding liver and more than 90% of the tumor has well defined margin; score 2, the echogenicity of the index tumor is slightly different from that of surrounding liver and more than 50% of the tumor has well defined margin; score 3, the index tumor is nearly iso-echoic to surrounding liver and less than 50% of the tumor has well defined margin; and score 4, definitely unidentifiable. The score was recorded in our radiologic report at the time of planning US. HCCs with scores 1 and 2 were regarded as fusion imaging-conspicuous lesions, whereas HCCs with scores 3 and 4 were regarded as fusion imaging-inconspicuous lesions since they were considered to have a risk of mistargeting or incomplete ablation. The radiologist also evaluated the feasibility of RFA for the index tumor, including the expected electrode path, adjacent organ vulnerable to thermal injury, tumor size, and heat-sink effect.13
In cases of HCC inconspicuous on fusion imaging (score 3 and 4), additional CEUS using Sonazoid was performed in order to accurately localize the index tumor at the time of planning US and/or RFA procedure. Before applying CEUS, we were able to estimate the location of the index tumor before contrast injection based on the findings of fusion imaging with B-mode US. Following this, a CEUS image was displayed with fused CT/MR images on the US monitor side-by-side.
CEUS was performed with the same US machine, with a 1-5 MHz convex probe. The contrast harmonic imaging (CHI) technique with a default setting of a mechanical index (MI) level of 0.24 was used. The liver was scanned at 15 frames per second. The focus point was located in the posterior margin of the liver. The contrast agent Sonazoid was used at a dose of 0.015 mL/kg by a manual bolus injection followed by a flush with 10 mL of normal saline via a peripheral venous line. Intermittent CEUS images were obtained during the following four phases: arterial (10-40 seconds after injection of Sonazoid), portal (60-90 seconds), delayed (3 minutes), and Kupffer phases (more than 10 minutes). If the index tumor was not identified on both arterial and Kupffer phases, Sonazoid was reinjected at least 10 minutes after the initial injection.14 Before the second injection of Sonazoid, microbubbles were destroyed by sweeping the liver with B-mode US with a high MI of 1.1. However, when the index tumor was identified at the Kupffer phase, the second injection was not routinely performed on any HCC nodules for the so called defect-reperfusion imaging since all tumors had arterial hypervascularity on either CT or MR images. Conspicuity score of the index tumor was reassessed by the aforementioned scoring system based on the findings of both arterial and Kupffer phase imaging. When the index tumor was inconspicuous even on fusion imaging with CEUS, treatments other than RFA were basically performed in most cases. If no other treatment option was available, blind RFA of the expected area of the liver with the help of fusion imaging with B-mode was attempted.
The index tumor was targeted with free hand technique on Kupffer-phase of CEUS image, when the tumor is seen as a perfusion defect on CEUS at the corresponding site of the fused CT/ MR image. We used an internally cooled length-adjustable electrode (Proteus Electrode; STARmed, Gyeonggi-do, KOREA, CO) or a separable clustered electrode (Octopus Electrode; STARmed, Gyeonggi-do, KOREA, CO) and a 200-W generator (VIVA RF System; STARmed, Gyeonggi-do, KOREA, CO). Artificial ascites was made before the RFA procedure whenever needed to improve the sonic window or to decrease the degree of collateral thermal injury to the adjacent organ. In these cases, the conspicuity score was reassessed after introducing artificial ascites, not before for both fusion imaging with B-mode US and fusion imaging with CEUS. Therefore, grading of the conspicuity score was not affected by the use of artificial ascites. Although the standard ablation time recommended by manufacturer was 12 minutes, RFA was terminated earlier if collateral thermal injury was suspected or the echogenic zone by the RFA cycle was large enough to achieve a sufficient ablative margin. The electrode path was cauterized during electrode removal at the end of the RFA procedure.
We evaluated the number of cases of initially inconspicuous HCCs that became conspicuous on fusion imaging with CEUS according to tumor size.
Therapeutic outcome and complications were assessed by three-phase liver CT within 12 hours after RFA according to a paper regarding standardization of image-guided tumor ablation.15 Whether the ablative margin was sufficient was evaluated on the immediate post-RFA CT by comparing pre-RFA CT/MR images in a side-by-side manner.16,17 Technical success was defined when the index tumor treated according to our treatment protocol was covered completely, which also indicates a complete elimination of enhancing areas within the entire tumor on immediate post-RFA CT.15 A major complication was defined as any event that resulted in substantial morbidity and disability, increasing level of care, or substantial lengthening of hospital stay.15 All other complications were regarded as minor. Primary technique effectiveness was assessed based on one-month follow-up CT images. Local tumor progression was defined when the foci of the untreated disease in the tumor that were initially considered to be completely ablated appear on follow-up CT or MR images.
During the study period, there were 60 HCCs inconspicuous on fusion imaging with B-mode US and CT/MR images. Among them, 30 HCCs were excluded due to the following reasons: (a) Fusion imaging-guided RFA was performed with the use of artificial ascites (n=4), (b) Treatments other than RFA were performed (n=11), (c) Follow-up without treatment was done (n=6), and (d) RFA was performed under the guidance of conventional CEUS mode, not combined use of fusion imaging and CEUS (n=9). Finally, a total of 30 patients (21 men and 9 women; mean age, 58.2 years; range, 39-70 years) with 30 HCCs were included (Fig. 1). All tumors were inconspicuous on fusion imaging with B-mode US and CT/MR images, and thus CEUS was applied in addition to fusion imaging. The baseline characteristics of 30 patients are summarized in Table 1. The most common cause of liver disease was hepatitis B virus infection (86.7%, 26/30). 10 (33.3%) patients had no history of HCC treatment, and the other 20 (66.7%) had undergone various treatments for HCCs. The tumor size was 1.2±0.3 cm (range, 0.6-1.7 cm). 16 HCCs were larger than 1 cm and the other 14 HCCs were equal or smaller than 1 cm.
All 14 HCCs ≤1 cm had typical imaging features of HCC on dynamic contrast-enhanced MR images. Most HCCs ≤1 cm had a previous history of HCC treatment (71.4%, 10/14), or showed an upward trend of serum alpha-fetoprotein (42.9%, 6/14). Moreover, they had a tendency of increasing size during the follow-up imaging studies (42.9%, 6/14).
The mean time interval between the date of CT/MR imaging and that of planning US was 16.8 days (range: 5-29 days). Before applying CEUS, the conspicuity score was graded as 3 in 12 (40.0%) HCCs and 4 in 18 (60.0%) HCCs on fusion imaging with B-mode US and CT/MR images (Table 2). Five (41.7%) lesions out of the 12 HCCs with a score of 3 were equal or smaller than 1 cm in diameter, and 9 (50.0%) lesions out of the 18 HCCs with a score of 4 were equal or smaller than 1 cm in diameter.
Of the 30 initially inconspicuous HCCs on fusion imaging with B-mode, 25 (83.3%) lesions became conspicuous on fusion imaging with CEUS (Table 2). Among the 12 HCCs with a score of 3 on fusion imaging with B-mode, the conspicuity core was increased in all 12 (100%) lesions on fusion imaging with CEUS [score 1 (n=12)]. Among the 18 HCCs with an initial score of 4 (Figs. 2 and 3), the conspicuity core was also increased in 14 (73.7%) lesions on fusion imaging with CEUS [score 1 (n=8), score 2 (n=5) and score 3 (n=1)]. Five lesions were still inconspicuous even on fusion imaging with CEUS. When the change of lesion conspicuity was analyzed according to tumor size, HCCs >1 cm (15/16, 93.8%) had a tendency to benefit from fusion imaging with CEUS more than HCCs ≤1 cm (11/14, 78.6%).
Among the 25 lesions which became conspicuous on fusion imaging with CEUS, index tumors were visualized as hyperenhancing nodules (n=21) in the arterial phase, hypoenhancing nodules (n=25) in the Kupffer phase, and in both (n=21). Among them, 23 lesions were considered RFA-feasible and thus treated by fusion imaging with CEUS-guided RFA (Figs. 2 and 3). The other two lesions were considered infeasible for RFA due to no safe electrode path and thus were managed with TACE. One lesion conspicuous on fusion imaging with CEUS during planning US became inconspicuous on fusion imaging with CEUS at the time of RFA procedure due to unexplained poor sonic window. The patient had a history of prior treatment with multiple TACE and additional TACE was difficult to perform due to hepatic arterial injury and hence was treated with RFA in a blind manner with the help of fusion imaging with CEUS. The fused images displayed with CEUS during RFA were as follows: hepatobiliary phase MR images (n=15), arterial phase MR images (n=7), or arterial phase CT images (n=1). Artificial ascites (n=4) was introduced before image fusion to enhance the sonic window (n=3) or to avoid thermal injury to the adjacent colon (n=1). The additional use of CEUS made it possible to localize the three index tumors that remained inconspicuous even after artificial ascites was used to enhance sonic window.
A single electrode was used in 19 cases, and the separable clustered electrode was used in 4 cases. The number of overlapping ablations was 1.7±1.0 (range, 1-5). The total ablation time was 7.1±2.5 min (range, 3-12 min). The remaining 5 lesions inconspicuous even on fusion imaging with CEUS were regarded as RFA-infeasible, thus were not treated with RFA.
Technical success, as assessed by CT obtained immediately after RFA, was achieved in 21 (91.3%) of 23 HCCs with a single RFA session. Technical failure was found in two patients including one patient who was treated with RFA of the expected area of the liver with the use of fusion imaging with CEUS in a blind manner. The size of these two HCCs was very small, 0.6 cm and 1.0 cm, respectively. In these two cases, although ablative margin was not sufficient on immediate post-RFA CT scan, residual unablated tumors were not apparent on initial assessment. Hence, instead of second RFA session, image follow-up was performed. On follow-up CT (4 months and 3 months, respectively), however, there were enhancing tumors at the ablative margin and these tumors were treated with additional RFA. On retrospective image review of immediate post-RFA CT images, residual enhancing tumors were suspected. Therefore, these two cases were considered as technical failure.
Liver abscess was found in one (4.3%, 1/23) patient during the follow-up period as a major complication. This patient underwent percutaneous catheter drainage and antibiotic treatment. Local tumor progression was developed in two patients during the follow-up period (mean, 4.3 months; range, 3.0-5.6 months) and was treated with either repeated RFA (n=1) or TACE (n=1).
Small HCCs are difficult to localize with B-mode US in patients with liver cirrhosis.18,19 However, these small HCCs with poor sonographic conspicuity can benefit from fusion imaging. According to previous studies,3,12,20 fusion imaging of B-mode US and CT/MR images is useful for localizing small HCCs. However, not all small HCCs can be identified on fusion imaging. Recently, Lee et al3 reported that 13 (13.1%) out of 99 HCCs (1-2 cm) were invisible even after image fusion. Although some of these inconspicuous tumors can be blindly ablated by inserting an electrode into the expected area of the liver using a peritumoral anatomic landmark on fusion imaging,21 a larger ablation zone would be necessary to preclude mistargeting or incomplete ablation. In addition, the operators who perform RFA procedure for inconspicuous HCCs would have higher stress level. To solve this problem, CEUS can be additionally applied to fusion imaging.
This study demonstrates that fusion imaging with CEUS is effective for percutaneous RFA of small HCCs (<2 cm) inconspicuous on fusion imaging with B-mode US. With additional use of CEUS, 83.3% (25/30) HCC nodules which were initially inconspicuous on fusion imaging with B-mode US became conspicuous. Consequently, 76.7% (23/30) lesions could be treated with percutaneous RFA guided by fusion imaging with CEUS. Moreover, accurate targeting of the tumor was facilitated on Kupffer phase imaging in which all the tumors were seen as defect lesions on CEUS at the corresponding site of fused CT/MR images compared to the well enhancing surrounding liver.
Typically, CEUS examination is displayed in the split-screen mode on a single monitor: a CEUS image on the left side and a background B-mode US image on the right side. However, when fusion imaging is available, CEUS can be displayed combined with fusion imaging: a CEUS image on the left side and a fused CT/MR image on the right side.22 The combined use of fusion imaging and CEUS has several advantages over CEUS mode alone for localizing small HCCs with poor sonographic conspicuity. With fusion imaging, the operator can estimate the location of the index tumor before contrast injection, making it easier to detect a nodule showing arterial enhancement during relatively short arterial phase after the first contrast injection. If the lesion seen as a hyperenhancing nodule during arterial phase present as a low echogenic defect lesion during Kupffer phase, we do not have to re-inject Sonazoid to do the so-called defect-reperfusion imaging.14 Therefore, the overall examination time of CEUS is reduced by avoiding the second injection of contrast. Consequently, the overall procedure time of CEUS-guided RFA would be substantially decreased.
In this study, at the time of targeting the tumor, we used the free hand technique, not the guiding device attached to the US transducer. This was because all 23 HCCs were seen as defect lesions in the Kupffer phase, which provide a long temporal window for us to insert an electrode. Hence, we were able to insert the electrode with high confidence. Having a wide temporal window of Kupffer phase seems to be one of the advantages of Sonazoid compared with other contrast agents.4,5 If the Kupffer phase is not available on CEUS, real-time targeting with the free hand technique would be much difficult due to the relatively short temporal window of the arterial phase. In fact, in a recent study in which SonoVue (Bracco, Milan, Italy) was used for CEUS-guided RFA,7 real-time targeting using the free hand technique was not performed due to the absence of the Kupffer phase. Instead, the authors performed CEUS prior to electrode insertion to preclude mistargeting of HCCs since the index tumors were small (mean size: 1.2 cm) and thus the tumors had poor conspicuity on B-mode US.
In this study, RFA was performed not only for HCCs >1 cm, but also for subcentimeter-sized HCCs. 78.6% (11/14) of subcentimeter-sized HCCs inconspicuous on fusion imaging became conspicuous on fusion imaging with CEUS (Table 2). Except two HCCs that were infeasible for RFA, 64.3% (9/14) of subcentimeter-sized HCCs inconspicuous on fusion imaging with B-mode US could be ablated under the guidance of fusion imaging with CEUS.
This study has several limitations. First, since this was a retrospective study, it suffers from selection bias. Second, in this study, all subcentimeter-sized HCCs were diagnosed by means of imaging studies according to the APASL guideline,11 not by histopathologic proof. However, until now, there are controversies in terms of imaging-based diagnosis and initiation of treatment for subcentimeter-sized HCCs between various guidelines.11,23,24 Nevertheless, this study may have worth investigating, since there is no prior data regarding whether fusion imaging with CEUS is useful for percutaneous RFA of subcentimeter-sized HCCs. We hope that experts will reach an agreement on the issue of imaging-based diagnosis of subcentimeter-sized HCC in the near future. Third, the conspicuity score of HCC nodules either on fusion imaging with B-mode US or on fusion imaging with CEUS was assessed by a single radiologist, not by consensus of multiple radiologists. Fourth, the follow-up period of this study was not sufficient to evaluate the therapeutic efficacy of RFA. However, in this study, we focused on the feasibility of fusion imaging with CEUS-guided RFA of HCCs inconspicuous on fusion imaging with B-mode US rather than long term therapeutic efficacy.
In conclusion, fusion imaging with CEUS and CT/MR images is very effective for percutaneous RFA of very early-stage HCCs inconspicuous on fusion imaging with B-mode US and CT/MR images. Therefore, the additional use of CEUS should be considered when fusion imaging alone is not satisfactory for localizing small HCCs.
REFERENCES
1. Lee MW, Rhim H, Cha DI, Kim YJ, Choi D, Kim YS, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma: fusion imaging guidance for management of lesions with poor conspicuity at conventional sonography. AJR Am J Roentgenol 2012;198:1438-1444. 22623560.


2. Minami Y, Chung H, Kudo M, Kitai S, Takahashi S, Inoue T, et al. Radiofrequency ablation of hepatocellular carcinoma: value of virtual CT sonography with magnetic navigation. AJR Am J Roentgenol 2008;190:W335-W341. 18492875.


3. Lee MW, Rhim H, Cha DI, Kim YJ, Lim HK. Planning US for percutaneous radiofrequency ablation of small hepatocellular carcinomas (1-3 cm): value of fusion imaging with conventional US and CT/MR images. J Vasc Interv Radiol 2013;24:958-965. 23796082.


4. Masuzaki R, Shiina S, Tateishi R, Yoshida H, Goto E, Sugioka Y, et al. Utility of contrast-enhanced ultrasonography with Sonazoid in radiofrequency ablation for hepatocellular carcinoma. J Gastroenterol Hepatol 2011;26:759-764. 21054516.


5. Numata K, Luo W, Morimoto M, Kondo M, Kunishi Y, Sasaki T, et al. Contrast enhanced ultrasound of hepatocellular carcinoma. World J Radiol 2010;2:68-82. 21160920.



6. Minami Y, Kudo M. Review of dynamic contrast-enhanced ultrasound guidance in ablation therapy for hepatocellular carcinoma. World J Gastroenterol 2011;17:4952-4959. 22174544.



7. Kim AY, Lee MW, Rhim H, Cha DI, Choi D, Kim YS, et al. Pretreatment evaluation with contrast-enhanced ultrasonography for percutaneous radiofrequency ablation of hepatocellular carcinomas with poor conspicuity on conventional ultrasonography. Korean J Radiol 2013;14:754-763. 24043968.



8. Maruyama H, Takahashi M, Ishibashi H, Okugawa H, Okabe S, Yoshikawa M, et al. Ultrasound-guided treatments under low acoustic power contrast harmonic imaging for hepatocellular carcinomas undetected by B-mode ultrasonography. Liver Int 2009;29:708-714. 18803588.


9. Kudo M, Hatanaka K, Maekawa K. Newly developed novel ultrasound technique, defect reperfusion ultrasound imaging, using sonazoid in the management of hepatocellular carcinoma. Oncology 2010;78(Suppl 1):40-45. 20616583.

10. Jang JY, Kim MY, Jeong SW, Kim TY, Kim SU, Lee SH, et al. Current consensus and guidelines of contrast enhanced ultrasound for the characterization of focal liver lesions. Clin Mol Hepatol 2013;19:1-16. 23593604.



11. Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int 2010;4:439-474. 20827404.



12. Kunishi Y, Numata K, Morimoto M, Okada M, Kaneko T, Maeda S, et al. Efficacy of fusion imaging combining sonography and hepatobiliary phase MRI with Gd-EOB-DTPA to detect small hepatocellular carcinoma. AJR Am J Roentgenol 2012;198:106-114. 22194485.


13. Rhim H, Choi D, Kim YS, Lim HK, Choe BK. Ultrasonography-guided percutaneous radiofrequency ablation of hepatocellular carcinomas: a feasibility scoring system for planning sonography. Eur J Radiol 2010;75:253-258. 19427152.


14. Kudo M, Hatanaka K, Maekawa K. Defect reperfusion imaging, a newly developed novel technology using Sonazoid in the treatment of hepatocellular carcinoma. J Med Ultrasound 2008;16:169-176.

15. Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD 3rd, Dupuy DE, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol 2009;20(7 Suppl):S377-S390. 19560026.


16. Nakazawa T, Kokubu S, Shibuya A, Ono K, Watanabe M, Hidaka H, et al. Radiofrequency ablation of hepatocellular carcinoma: correlation between local tumor progression after ablation and ablative margin. AJR Am J Roentgenol 2007;188:480-488. 17242258.


17. Kim YS, Rhim H, Cho OK, Koh BH, Kim Y. Intrahepatic recurrence after percutaneous radiofrequency ablation of hepatocellular carcinoma: analysis of the pattern and risk factors. Eur J Radiol 2006;59:432-441. 16690240.


18. Lee MW, Kim YJ, Park HS, Yu NC, Jung SI, Ko SY, et al. Targeted sonography for small hepatocellular carcinoma discovered by CT or MRI: factors affecting sonographic detection. AJR Am J Roentgenol 2010;194:W396-W400. 20410384.


19. Rhim H, Lee MH, Kim YS, Choi D, Lee WJ, Lim HK. Planning sonography to assess the feasibility of percutaneous radiofrequency ablation of hepatocellular carcinomas. AJR Am J Roentgenol 2008;190:1324-1330. 18430851.


20. Makino Y, Imai Y, Ohama H, Igura T, Kogita S, Sawai Y, et al. Ultrasonography fusion imaging system increases the chance of radiofrequency ablation for hepatocellular carcinoma with poor conspicuity on conventional ultrasonography. Oncology 2013;84(Suppl 1):44-50. 23428858.


21. Song KD, Lee MW, Rhim H, Cha DI, Chong Y, Lim HK. Fusion imaging-guided radiofrequency ablation for hepatocellular carcinomas not visible on conventional ultrasound. AJR Am J Roentgenol 2013;201:1141-1147. 24147489.


22. Numata K, Fukuda H, Morimoto M, Kondo M, Nozaki A, Oshima T, et al. Use of fusion imaging combining contrast-enhanced ultrasonography with a perflubutane-based contrast agent and contrast-enhanced computed tomography for the evaluation of percutaneous radiofrequency ablation of hypervascular hepatocellular carcinoma. Eur J Radiol 2012;81:2746-2753. 22197088.


Figure 1
Flow chart of this study. *Contrast-enhanced ultrasonography (CEUS) was applied additionally during radiofrequency ablation (RFA) of hepatocellular carcinoma (HCC). †In the early study period, the conventional CEUS mode in which CEUS images and B-mode ultrasound (US) images appeared side-by-side was applied, and not combined with fusion imaging. These cases were excluded from this study. ‡One lesion conspicuous on fusion imaging with CEUS during planning US became inconspicuous on fusion imaging with CEUS at the time of the RFA procedure due to an unexplained poor sonic window, and was treated with RFA in a blind manner with the help of fusion imaging with B-mode US using the peritumoral vessels as anatomic landmarks. §Two lesions became conspicuous on fusion imaging with CEUS, but were not considered feasible for RFA. They were treated by transcatheter arterial chemoembolization (TACE). ∥Five lesions that remained inconspicuous even on fusion imaging with CEUS were treated by TACE (n=4) or surgery (n=1).
Figure 2
A 74-year-old man with an HCC in segment 6 of the liver. (A) Arterial-phase magnetic resonance (MR) image showing a 1.0-cm HCC (arrow) in segment 6. (B) After applying the fusion imaging technique, an index tumor is definitely unidentifiable at the corresponding site (black arrow) on the fused MR image. The surrounding liver has a heterogeneous echo texture; it is thus difficult to detect a true index tumor. Therefore, the conspicuity score was graded as 4. (C) On fusion imaging with CEUS using Sonazoid, a hypervascular mass (black arrow) is clearly identified on the arterial-phase image at the corresponding site (black arrow) of the fused MR image. (D) On Kupffer-phase imaging, the index tumor (black arrow) is seen as a perfusion defect at the corresponding site (black arrow) of the fused MR image. (E) An electrode (arrowheads) was inserted into the index tumor (black arrow) under the guidance of simultaneous display of CEUS and fused MR images. (F) Arterial-phase computed tomography (CT) image obtained immediately after RFA showing technical success with a sufficient ablative margin (arrowheads).
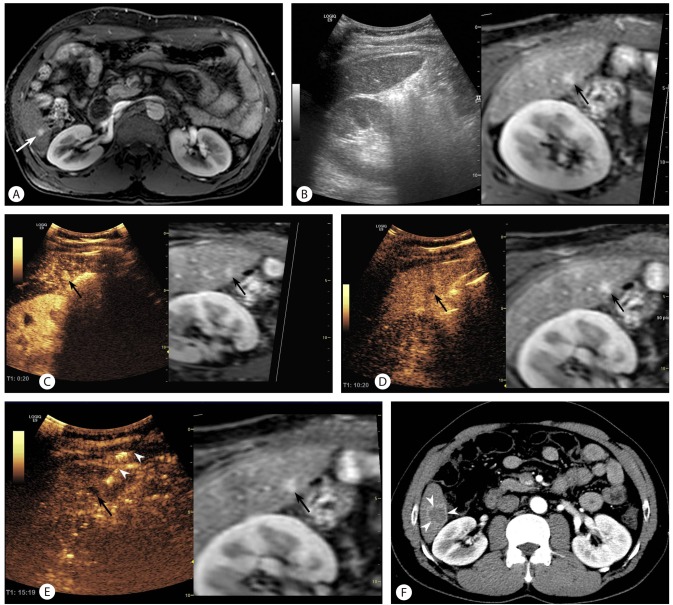
Figure 3
A 67-year-old man with an HCC in segment 8 of the liver, and history of RFA and TACE. (A) Arterial-phase MR image showing a 1.0-cm-sized HCC (arrow) in segment 8. (B) On hepatobiliary-phase MR imaging, the lesion is seen as a small hypointense nodule (arrow). (C) Percutaneous RFA was performed under fusion imaging guidance. Artificial ascites (white asterisks) were introduced to improve the sonic window. On B-mode US (left image), a low echoic area (arrowheads) can be seen at the corresponding site of the fused MR image (right image). However, the lesion looks much larger on the US image (black arrow) than on the fused MR image. Since the boundary of the tumor is not demarcated at all, the conspicuity score of the lesion was graded as 4. A black asterisk indicates the previous ablation zone. (D) On arterial-phase imaging using Sonazoid, a small enhancing lesion (arrow) is clearly identifiable within the low echoic area (arrowheads), which was seen on B-mode US on previous fusion imaging (C). (E) At approximately 6 minutes after contrast administration, the index tumor (arrow) could be clearly identified as a perfusion defect at the corresponding site (black arrow) of the fused MR image. Therefore, the conspicuity score of the index tumor was graded as 1 on fusion imaging with CEUS. (F) An electrode (arrowheads) was inserted into the index tumor (arrow) under the guidance of fusion imaging with CEUS. (G) An arterial-phase CT image obtained immediately after RFA reveals technical success with a sufficient ablative margin (arrowheads).

Table 1.
Baseline characteristics of the 30 patients with 30 hepatocellular carcinomas (HCCs)
Table 2.
Changes in the conspicuity scores of the 30 HCCs after adding contrast-enhanced ultrasonography to fusion imaging
- TOOLS



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




