| Clin Mol Hepatol > Volume 20(3); 2014 > Article |
ABSTRACT
Radio-frequency ablation (RFA) is a curative treatment for hepatocellular carcinoma (HCC). Percutaneous RFA has been shown to be beneficial for patients with small renal cell carcinoma (RCC) lacking indications for resection. We experienced the case of a 53-year-old male who had conditions that suggested HCC, RCC, and alcoholic liver cirrhosis. Abdominal contrast-enhanced computed tomography (CT) and magnetic resonance image showed liver cirrhosis with 2.8 cm ill-defined mass in segment 2 of the liver and 1.9 cm hypervascular mass in the left kidney. These findings were compatible with the double primary cancers of HCC and RCC. Transarterial chemoembolization (TACE) was performed to treat the HCC. After the TACE, a focal lipiodol uptake defect was noticed on a follow up CT images and loco-regional treatment was recommended. Therefore, we performed RFAs to treat HCC and RCC. There was no evidence of recurrence in the follow up image after 1 month.
The coexistence hepatocellular carcinoma (HCC) and renal cell carcinoma (RCC) is extremely rare, and only a few case reports could be found in the literature.1
Radiofrequency ablation (RFA) has been shown to be safe and successful for local tumor control in patients with HCC and is increasingly used for HCC patients.2,3,4 Currently, RFA and cryoablation are considered alternatives to a partial nephrectomy in patients unable to undergo surgery.5 These treatment options offer several benefits compared to a nephrectomy, including a lower complication rate, shorter hospital stay, less ischemic damage, and the potential for outpatient management of the patient.5
We present a case of synchronous HCC and RCC treated with RFA and a review of the literature.
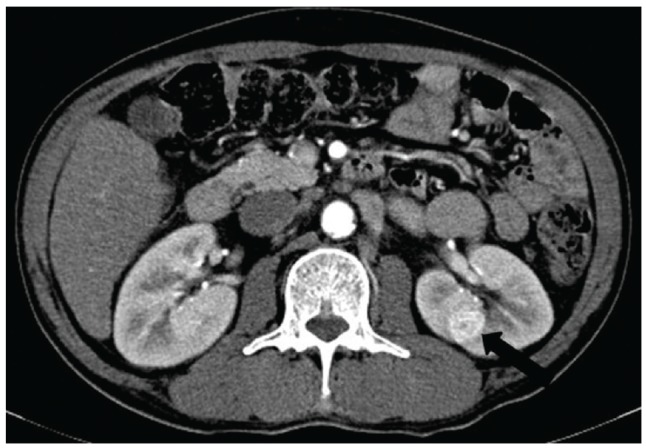
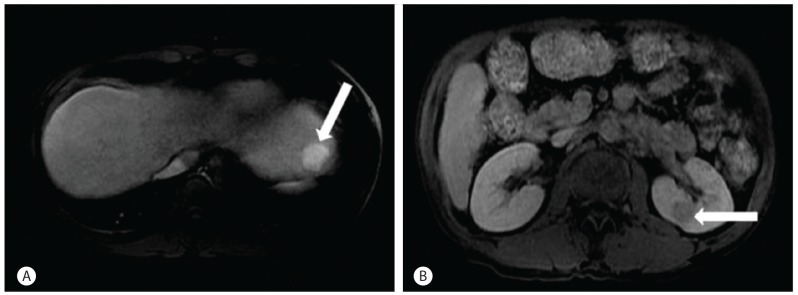
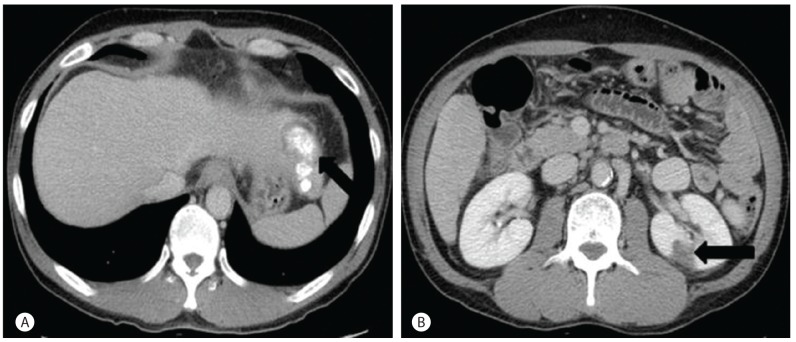
A-53-year-old man visited our hospital with general weakness. He was referred to our hospital because of condition that suggested HCC, RCC, and alcoholic liver cirrhosis. His past medical history only included diabetes mellitus (DM). He has no family history of disease. The patient has a 30 pack-year smoking history and would drink so ju (a Korean distilled spirits), approximately 2-3 bottles daily for the previous 3 months. The serum bilirubin was 2.6 mg/dL, serum albumin was 2.9 g/dL, and prothrombin time (INR; International Normalized Ratio) was 1.26. A small amount of ascites was observed. Hepatic encephalopathy was not observed (Child-Pugh score 9, stage B). Abdominal contrast-enhanced computed tomography (CT) and magnetic resonance image (MRI) showed liver cirrhosis with 2.8 cm ill-defined mass in segment 2 of the liver and 1.9 cm hypervascular mass in left kidney. An enhancing mass, approximately 2.8 cm, without an apparent border in the arterial phase and washed out in delayed phase was noticed in S2 of the liver (Fig. 1). A mass with a thickened smooth wall, approximately 1.9 cm, in the enhancing arterial phase in left kidney was noticed in the arterial phase in a CT image (Fig. 2). These findings were compatible with double primary cancers of HCC and RCC. There was no evidence of lymph node or distant metastasis. In S2 of the liver, a hypervascular mass, approximately 2.8 cm was noticed in the T2 weighted image in the MRI and an approximate 1.9 cm mass in the left kidney was noticed in a T1 weighted image in the MRI (Fig. 3). The serum alpha fetoprotein (AFP) was 6.93 ng/mL and the serum protein induced by vitamin K absence/antagonist-II (PIVIKA-II) was 23 mAU/mL. The HBsAg, Anti-HBs and Anti-HCV were all negative. Transarterial chemoembolization (TACE) was performed to treat the HCC. Two days after the TACE, the focal lipiodol uptake defect was noticed in follow up CT images and loco-regional treatment was recommended. No other significant abnormal hypermetabolic lesion suggesting malignancy was noticed on positron emission tomography-CT (PET-CT). Therefore, we performed RFAs to treat HCC and RCC. There was no significant complication after the procedure. One month later, follow-up CT images were taken. There was no evidence of recurrence (Fig. 4).
To our knowledge, this is first report of a double primary HCC and RCC case that were both treated with RFA. Synchronous early primary cancers are rare. One study showed an incidence of 3.7% of synchronous cancers related to RCC.1 Such malignancies include urological cancers, esophageal carcinomas, colorectal carcinomas, lung cancer, breast cancer, gynecological cancer, sarcoma and non-Hodgkin's lymphoma.6 The occurrence of synchronous RCC and HCC is extremely rare.1 HCC can be diagnosed from a CT image with a hypervascular mass in the arterial phase and washout in the portal venous or delayed phases.7 The typical imaging characteristics of HCC on MRI are a large (>2 cm) hyperintense lesion on T2-weighted images.8 The spiral CT has significantly improved the imaging of renal masses by decreasing the volume averaging artifacts and respiratory misregistration artifacts and allowing image acquisition during optimal contrast enhancement.9 Single- and multidetector CT have helped refine the diagnostic work-up of renal masses by allowing for image acquisition in various phases of renal enhancement after the intravenous administration of a single bolus of contrast material.9 The renal parenchyma enhances homogeneously, allowing for the best opportunity for discrimination between the normal renal medulla and masses.9 Most RCCs have the tendency to show isointensity to hypointensity on T1-weighted images and hyperintensity on T2-weighted images, although large RCCs tend to show heterogeneous signal intensity reflecting necrosis and hemorrhage.10 Our specific CT and MRI findings were correctly indicated in the diagnostic criteria of the HCC and RCC. A non-invasive diagnosis was established by one imaging technique in nodules above 2 cm showing the HCC radiological hallmark and two coincidental techniques with nodules of 1-2 cm in diameter (CT, MRI and US-contrast). AFP levels were dropped from the diagnostic scheme.7
RFA has been shown safe and successful for local tumor control in patients with HCC and is increasingly used for HCC patients.2,3,4 A single RFA treatment can necrotize approximately 3 cm area.11 Currently, 3 or fewer tumors that are 3 cm or smaller are generally indicated for RFA.11 For tumors larger than 3 cm in diameter, TACE is frequently performed first, followed by additional RFA.12 Lipiodol TACE-preceded by RFA is aiming at the treatment of local residual and microsatellite lesions and micro-invasion and ensuring an accurate margin by lipiodol injection.12 Blood flow promotes heat loss, and reducing or eliminating blood flow during the RFA procedure is known to increase the volume of the ablative zone.13 Because HCCs are supplied almost entirely by the hepatic arteries, it seems reasonable to perform RFA after chemoembolization.13 TACE and RFA were shown to be as effective as a liver resection for HCC in a recent study, with similar 1-, 3-, and 5-year overall and recurrence-free survival rates.13
In cases of RCC, radical or partial nephrectomy is the standard treatment for localized RCC.14 Since it was first applied in 1997, renal RFA has become increasingly more accepted during the past few years.14 RFA has been used as a nephron sparing alternative to a partial nephrectomy, with good early results.14
Recent report shows that RFA is an effective minimally invasive therapy for the treatment of cT1a RCC, yielding equivalent long-term oncologic outcomes to nephron-sparing surgery.15 A characteristic of RFA is its cooling effect on the blood flow, which has a considerable effect on the outcome of RFA treatment.16 The potential advantages of RFA over surgery include: having minimal invasiveness, avoiding damage to adjacent tissues, preserving the surrounding renal parenchyma, and reducing the complication rate, resulting in a shorter hospital stay.17 But liver cirrhosis patients have an inhibitive risk to perform either a biopsy of the RCC or its resection. An RFA of malignant tumors has been a viable option in patients with primary and metastatic liver lesions, who are not candidates for surgery.16
In conclusion, coexistence of RCC and HCC is extremely rare. HCC and RCC can be diagnosed by the specific findings of a CT or MRI. Currently RFA has been used for the treatment of HCC or RCC with an appropriate indication. This is first report of synchronous HCC and RCC treated with RFA. Regularly scheduled follow-up is necessary to confirm the treatment results.
REFERENCES
1. Garcia JH, Coelho GR, Cavalcante FP, Valença JT Jr, Brasil IR, Cesar-Borges G, et al. Synchronous hepatocellular carcinoma and renal cell carcinoma in a liver transplant recipient: a case report. Transplantation 2007;84:1713. 18165787.


2. Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology 2010;52:762-773. 20564355.


3. Guimaraes M, Uflacker R. Locoregional therapy for hepatocellular carcinoma. Clin Liver Dis 2011;15:395-421. 21689621.


4. Germani G, Pleguezuelo M, Gurusamy K, Meyer T, Isgro G, Burroughs AK. Clinical outcomes of radiofrequency ablation, percutaneous alcohol and acetic acid injection for hepatocelullar carcinoma: a meta-analysis. J Hepatol 2010;52:380-388. 20149473.


5. Heuer R, Gill IS, Guazzoni G, Kirkali Z, Marberger M, Richie JP, et al. A critical analysis of the actual role of minimally invasive surgery and active surveillance for kidney cancer. Eur Urol 2010;57:223-232. 19853989.


6. Papalampros AE, Petrou AS, Mantonakis EI, Evangelou KI, Giannopoulos LA, Marinos GG, et al. Coexistence of a colon carcinoma with two distinct renal cell carcinoma. J Med Case Rep 2011;5:134. 21463521.




7. European Association For The Study Of The Liver. European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-943. 22424438.


8. Witjes CD, Willemssen FE, Verheij J, van der Veer SJ, Hansen BE, Verhoef C, et al. Histological differentiation grade and microvascular invasion of hepatocellular carcinoma predicted by dynamic contrast-enhanced MRI. J Magn Reson Imaging 2012;36:641-647. 22532493.


9. Sheth S, Scatarige JC, Horton KM, Corl FM, Fishman EK. Current Concepts in the Diagnosis and Management of Renal Cell Carcinoma: Role of Multidetector CT and Three-dimensional CT. Radiographics 2001;21:S237-S254. 11598260.


10. Shinmoto H, Yuasa Y, Tanimoto A, Narimatsu Y, Jinzaki M, Hiramatsu K, et al. Small renal cell carcinoma: MRI with pathologic correlation. J Magn Reson Imaging 1998;8:690-694. 9626888.


11. Ansari D, Andersson R. Radiofrequency ablation or percutaneous ethanol injection for the treatment of liver tumors. World J Gastroenterol 2012;18:1003-1008. 22416173.



12. Arii S, Sata M, Sakamoto M, Shimada M, Kumada T, Shiina S, et al. Management of hepatocellular carcinoma: report of Consensus Meeting in the 45th Annual Meeting of the Japan Society of Hepatology. Hepatol Res 2010;40:667-685. 20633193.


13. Yamakado K, Nakatsuka A, Takaki H, Yokoi H, Usui M, Sakurai H, et al. Early-stage hepatocellular carcinoma: radiofrequency ablation combined with chemoembolization versus hepatectomy. Radiology 2008;247:260-266. 18305190.


15. Olweny EO, Park SK, Tan YK, Best SL, Trimmer C, Cadeddu JA. Radiofrequency ablation versus partial nephrectomy in patients with solitary clinical T1a renal cell carcinoma: comparable oncologic outcomes at a minimum of 5 years of follow-up. Eur Urol 2012;61:1156-1161. 22257424.


Figure 1
A contrast-enhanced abdominal CT shows a 2.8 cm sized mass in segment 2 of the liver with enhancement during the arterial phase and washed out during the delayed phase. (A) Arterial phase. (B) Delayed phase.
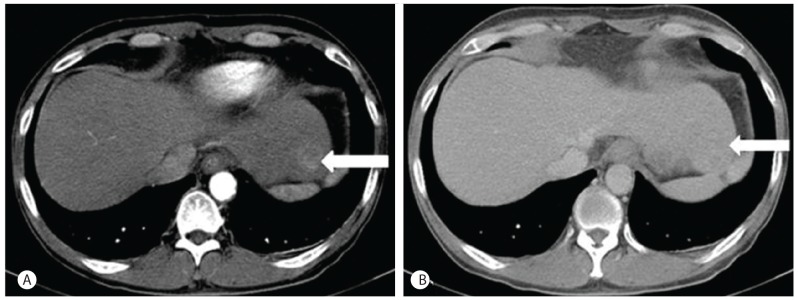
Figure 2
Contrast-enhanced abdominal CT shows a 1.9 cm sized mass in the mid-pole of the left kidney with enhancement during the arterial phase.
- TOOLS
-
METRICS

- Related articles
-
Development and prognosis of hepatocellular carcinoma in patients with diabetes2023 January;29(1)
Can hepatocellular carcinoma recurrence be prevented after liver transplantation?2021 October;27(4)
Exophytic combined hepatocellular carcinoma and cholangiocarcinoma2012 December;18(4)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



