INTRODUCTION
Glycogenic hepatopathy (GH) was first identified at 1930 and it is characterized by elevated liver enzyme, hepatomegaly, and glycogen accumulation within hepatocytes and it can be found in patients with type I Diabetes Mellitus (DM) with poorly controlled blood sugar.1,2 Clinically, GH has been known to have benign nature; however it is difficult to distinguish with nonalcoholic fatty liver disease (NAFLD) which could progress into advance liver disease without histological confirmation.2,3 Recently, we have diagnosed three cases of GH presented marked serum transaminases elevation more than 30 times of upper normal range as like acute hepatitis with poorly controlled type I DM history. Since the first report, about 15 cases suspected as GH have been described in adults over the world. In Korea, before this report, only 2 cases of GH have been reported for adults, however, one case showed only mild AST elevation (65 U/L) and normal range of ALT and the other case showed mild liver enzyme elevation during diabetic ketoacidosis (DKA) management.4,5 On the contrary, our three cases of GH in this report showed dramatic elevation of liver enzyme as like acute hepatitis and sustained fluctuation as like relapsing hepatitis, and these findings are very rare and unique even among the all reports of GH over the world. So, we present these cases with literature review.
CASE REPORT
Case 1
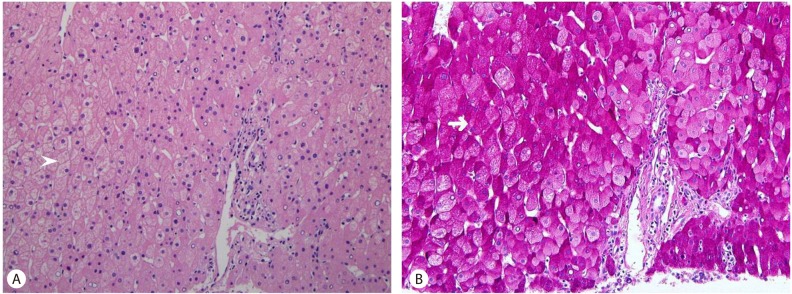
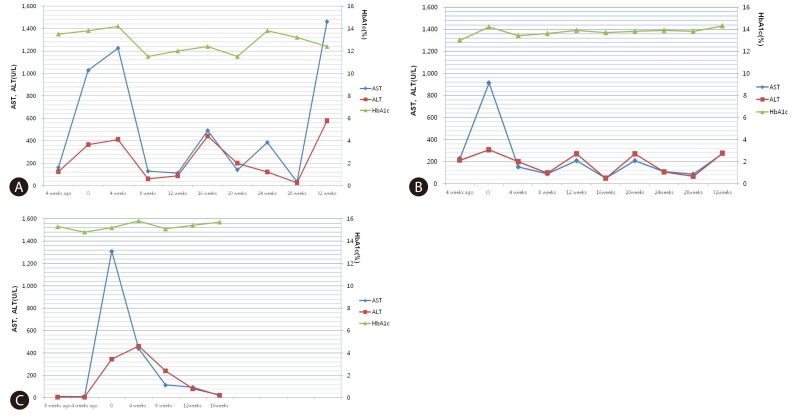
A 22-year old female was referred to a hepatologist for evaluation of elevated liver enzymes. She had haven Type I DM for 8 years. When she visited our hospital one month ago, her liver function test had showed abnormal aspartate aminotransferase (AST) and alanine aminotransferase (ALT) as 169 U/L (normal value <35) and 172 U/L (normal value <35), respectively. Four weeks later, at the time of the referral, her AST and ALT were elevated strikingly as 1028 U/L and 365 U/L. Other laboratory findings were total bilirubin (TB) 0.5 mg/dL (normal value <1.3), Alkaline phosphatase (ALP) 346 U/L (normal value <85), ╬│-glutamyl transpeptidase (╬│-GT) 321 U/L (normal value <40), hemoglobin A1c (HbA1c) 13.8% (normal value <6), triglyceride(TG) 160 mg/dL, high-density lipoprotein (HDL) 114 mg/dL creatine kinase (CK) 127 UL (normal value <220). Auto-antibody including anti-nuclear antibody (ANA), smooth muscle antibodies (ASMA) and mitochondrial antibodies (AMA) were all negative. Thyroid function test and serum ceruloplasmin were also normal. The serologic findings for viral infection such as IgM anti-HAV, HBsAg, anti-HCV, HCV-RNA, cytomegalovirus and Epstein-Barr virus were also all negative. Her height, weight, body mass index (BMI) and waist circumference were 165.1 cm, 50.8 kg, 18.6 kg/m2 and 72 cm respectively. Abdominal Ultrasonography (US) showed just a mild fatty change of liver and mild hepatomegaly (Fig. 1). She was not an alcohol or drug consumer and was not taking any medication except insulin. In addition, she did not show any symptom and sign compatible with acute hepatitis. So, we recommend her for a liver biopsy to elucidate the cause of acute elevation of liver enzyme. In hematoxylin and eosin (H&E) stain, liver biopsy showed normal liver architecture with no evidence of hepatocellular injury, inflammation, fibrosis, or steatosis. However, the hepatocytes were diffusely swollen with pale cytoplasm. The periodic acid-Schiff (PAS) staining showed abundant glycogen accumulation within hepatocytes. So, histologically, it was diagnosed as a glycogenic hepatopathy (Fig. 2). Eight weeks later, her transaminases were drooped suddenly without any special management. After this event, although we tried to control her sugar level strictly, her sugar control was still poor and HbA1c was maintained above 11% with mild fluctuation. Until now, her liver enzymes also have shown severe fluctuation continuously as like relapsing hepatitis (Fig. 3A).
Case 2
A 26-year year old female was referred to a hepatologist for evaluation of elevated liver enzymes. She had haven Type I DM for 9 years. Her glycemia had been poorly controlled with several episodes of DKA & DM gastropathy. When she visited our hospital 4 weeks ago, her AST and ALT had shown relatively mild elevation as 218 and 213 U/L, respectively. At the time of the referral, her AST and ALT were elevated strikingly to 914 U/L and 307U/L. Other laboratory findings were TB 0.5 mg/dL, ALP 189 U/L, ╬│-GT 294 U/L, HbA1c 12.9%, TG 185 mg/dL, HDL 112 mg/dL CK 112 UL. ANA, ASMA and AMA were all negative. Thyroid function test and serum ceruloplasmin were also normal. The serologic findings for viral infection were also all negative. Her height, weight, body mass index (BMI) and waist circumference were 154.3 cm, 56.3 kg, 23.6 kg/m2 and 79 cm, respectively. Abdominal US showed just a mild fatty change of liver. She had no history of taking alcohol or drug except insulin. In addition, she did not show any symptom and sign compatible with acute hepatitis. In liver biopsy, histological findings were compatible with GH. Four weeks later, her transaminases dropped spontaneously without any special management. After this event, her glycemic control has been still poor and HbA1c has been maintained above 13%, liver enzyme levels have shown continuous fluctuation (Fig. 3B).
Case 3
A 20-year old female with type I DM during 10 years was referred to a hepatologist for elevated liver enzymes. When she visited our hospital 8weeks ago, her AST and ALT levels were within normal range as 13 and 9 U/L, respectively. At the time of the referral, her AST and ALT were elevated strikingly to 1,310 U/L and 346 U/L. Other laboratory finding such as TB (0.2 mg/dL), ALP (132 U/L), ╬│-GT (15 U/L), TG (112 mg/dL), HDL (36 mg/dL), CK 86 UL, thyroid function test, serum ceruloplasmin level were all normal. ANA, ASMA and AMA were also negative. The serologic findings for viral infection were also all negative. There was no history of taking alcohol or drug except insulin. Her glycemic control had been poor and HbA1c was 13.6%. Her height, weight, body mass index (BMI) and waist circumference were 149.8 cm, 47.1 kg and 21 kg/m2, respectively. Abdominal US showed just a mild fatty change of liver. As former two cases, she did not show any symptom and sign compatible with acute hepatitis and GH was confirmed by liver biopsy. During the follow up, her glycemic control has been still poor and HbA1c has been maintained above 15%. However, her liver enzyme levels have shown dramatically down and maintenance within normal range (Fig. 3C).
DISCUSSION
We reported three cases of GH which were associated with type I diabetes mellitus, causing a clinical picture of acute and relapsing hepatitis. GH can cause severe, but reversible elevations of serum transaminase levels in patients with poorly controlled type I diabetes due to hepatic glycogen accumulation. Long-standing hyperglycemia, over insulin use, glucose given to control hypoglycemia and increased concentrations of the counter regulatory hormones have been known as risk factors for hepatic glycogen accumulation in patients with type I DM by their actions on the glycogen phosphorylase and synthase enzymes.6-8
The typical clinical findings of GH are hepatomegaly and elevated liver enzymes, especially transaminases.2,3,9-14 However, there is no histological evidence that suggests the enzyme elevations are due to liver necrosis, so their elevation is considered as a result of hepatocyte's membrane injury to cause enzyme "leakage" instead of cell death. However, the exact mechanism as well as prevalence has not been well known. Liver biopsy is helpful to confirm the diagnosis of GH. Histological features in GH are characterized by large, swollen, glycogen-laden hepatocytes and glycogenated nuclei without significant fatty change, inflammation, lobular spotty necrosis or fibrosis.1
The abnormality of transaminase in GH has been known to be easily treatable by improvement of glycemic control in a short time within 2 to 14 weeks.4,5,12,15 However, as seen in our case, the changes of liver enzyme levels showed somewhat different pattern compared with previous reported cases. The sudden elevation and dropping of transaminase did not showed any definitive correlation with the glycemic control (the change of HbA1C) and continuous fluctuation of transaminase as like relapsing hepatitis in case 1 and 2 is also unusual pattern compared to previous reports. In addition, there was no insulin dose-up even in acute flare-up phase of transaminase. So, it suggests that the changes of transaminases can be related with not only poor glycemic control or insulin therapy but also another factor which has not been clarified. In addition, in all cases, the AST dominant elevation compared to ALT is also impressive finding. Therefore, to elucidate the risk factors and more complete pathophysiology, more advanced systemic review and research have to be performed.
Clinically, to distinguish GH from NAFLD is important, because as mentioned above, the prognosis of both diseases are different. GH has been known to have benign nature whereas NAFLD could progress into advance liver disease such as cirrhosis or hepatocellular carcinoma.2,3 Including our cases, overall GH in diabetic patients with hepatomegaly and abnormal liver enzymes within 5-10 times of upper normal range have often been assumed to have NAFLD rather than GH in the initial diagnostic process. GH is a rare condition and many clinicians are unaware of it if no biopsy is obtained.16 As mentioned above, especially in case of mild transaminases elevation in diabetic patients, liver biopsy is essential in differential diagnosis of GH with NAFLD. In addition, US finding is not helpful in distinguishing NAFLD from GH because both fatty liver and glycogen overload could show similar US finding as mild hyperechoic hepatic parenchyma.3,6,10,11 Consequently, the condition is often misdiagnosed and it result in wasting medical resources.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




