Dear Editor,
Philadelphia chromosome-negative myeloproliferative neoplasms (MPNs) which include polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF) are characterized by erythrocytosis, leukocytosis, thrombocytosis, bone marrow hypercellularity, splenomegaly, and extramedullary hematopoiesis [
1]. A rare complication of Philadelphia-negative MPNs is portal hypertension (PHT). Previous reports have indicated that the incidence of PHT in patients with MPN is approximately 7% to 18% [
2]. The first manifestation of the disease may be complications of PHT, such as bleeding gastroesophageal varices (GEVs) [
3]. Typically, endoscopic variceal ligation and sclerotherapy is used to control GEVs, and a transjugular intrahepatic portosystemic shunt (TIPS) is applied for variceal bleeding or refractory ascites [
4].
MPNs are often associated with Janus kinase 2 (JAK2) V617F mutations [
5]. JAK2 is a nonreceptor tyrosine kinase expressed by all hematopoietic stem cells; it relays the signal to induce cell proliferation when cells are stimulated by external cytokines or growth factors [
5,
6]. The JAK2 V617F mutation leads to the constitutive phosphorylation of JAK2 and stimulates the cell proliferation cascade and the clonal proliferation of hematopoietic cells [
6]. The JAK2 V617 mutation may be a risk factor for splanchnic circulation thrombosis in patients with MPN and subclinical MPN, although the detailed mechanism has not been elucidated [
2]. An increased JAK2 mutation frequency in splanchnic circulation thrombosis or extramedullary hematopoiesis is associated with a high PHT incidence in patients with MPN. However, it is not clear whether specific subgroups of patients with MPN are more susceptible to the development of PHT or whether the JAK2 V617F mutation is associated with PHT in patients with MPN.
In this retrospective study with a large cohort of MPNs, we evaluated the clinical characteristics of patients with PHT complicated by GEVs and Philadelphia-negative MPNs. Most previous reports of PHT in MPN are case reports or case series, and detailed clinical characteristics of patients are generally lacking [
1,
6-
8]. The mechanisms underlying PHT in MPNs are still unclear. Increased blood flow into the portal system through the enlarged spleen is one of the primary causes of the development of PHT. The intrahepatic obstruction of the portal system due to myeloid metaplasia or sinusoidal change also increases portal pressure [
9]. Thrombosis of the portal vein due to blood vessel endothelium damage also induce PHT [
10].
We designed a retrospective single-center study. This study was approved by the Institutional Review Board of Seoul St. MaryŌĆÖs Hospital (KC19RESI0476). The medical records of all patients diagnosed with MPNs at Seoul St. MaryŌĆÖs Hospital between January 2009 and December 2018 were reviewed.
BCR-ABL1-negative MPNs primarily include PV, ET, PMF, and myeloproliferative neoplasm unclassified (MPN-U). The clinical diagnosis of PV, ET, PMF, and MPN-U was conducted in accordance with the 2016 WHO classifications [
11].
Normal hepatic venous pressure gradient (HVPG) is typically 1 to 5 mmHg, and significant PHT is considered as an increase in HVPG Ōēź10 mmHg, leading to the development of complications of PHT [
12]. Owing to the inability to measure HVPG or to perform endoscopy, PHT was evaluated by the existence of GEVs, as verified by abdominal computed tomography (CT). An esophageal varix was radiologically defined as an enhancing nodular tubular structure protruding into the esophageal lumen. A previous report demonstrated that CT showed a 90% sensitivity in the detection of large (>5 mm in diameter) GEVs [
13]. In our study, to increase the specificity of clinically significant GEV detection, the threshold diameter for esophageal varix was set as 5 mm on abdominal CT. Data were collected at the time of the abdominal CT. Quantitative variables are expressed as medians (interquartile range), whereas percentages are reported for qualitative data. Comparisons between groups were performed using the Mann-Whitney test. Values of
P<0.05 were considered statistically significant.
Two hundred and twenty eight patients with MPN underwent contrast-enhanced abdominal CT at least once between January 2009 and December 2018 (
Table 1,
2). Among these 228 patients, 50 (21.9%) were diagnosed with PV, 72 patients (31.6%) had ET, 84 patients (36.8%) had PMF, and 22 patients (9.7%) did not meet the criteria for these three diseases and were classified as MPN-U. A total of 11 patients among 228 patients had GEVs, as confirmed by abdominal CT. A total of 130 out of 228 patients had the JAK2 V617F mutation and all patients with GEV had the JAK2 V617F mutation (57% vs. 100%,
P=0.003). The incidence of the JAK2 V617F mutation in each group was as follows: 68% (34/50), PV; 52% (37/72), ET; 56% (47/84) PMF; 45% (10/22), MPN-U.
Among 11 patients having GEVs, five had PV, two had ET, three had PMF, and the remainder were classified as having MPN-U. The median age was 60, and five subjects were male. The median spleen size was 16.9 cm. No patients had chronic viral hepatitis or other chronic liver diseases such as hemochromatosis or autoimmune hepatitis (
Table 1,
2). Three had portal vein thrombosis without abnormalities in coagulation factor assays. All these patients were evaluated for the liver function (international normalized ratio, albumin, total bilirubin, presence of ascites, and presence of hepatic encephalopathy), and were categorized into ChildPugh class A, indicating that the liver function was well-preserved (
Table 1,
2). Three patients had variceal bleeding, which had not been lethal and controlled by endoscopic ligation. Seven patients received hydroxyurea and two patients received ruxolitinib (a JAK1, 2 inhibitor), but both of the medication resulted in no changes in variceal size by CT imaging.
A 53-year-old patient was diagnosed with PMF in 2016 at Seoul St. MaryŌĆÖs Hospital. Ruxolitinib treatment was started after the diagnosis. On August 4, 2017, he visited the emergency room presenting with hematemesis, and esophageal varix bleeding was documented. The bleeding was successfully stopped by endoscopic ligation. Prominent varix formation (F3) was detected and TIPS was performed on August 10, 2017. In September of 2017, he developed hepatic encephalopathy and visited the hospital again. He and his family decided to undo the shunt and TIPS closure was performed on September 25, 2017. Within 1 month of the procedure, the patient presented with recurrent hematemesis. Esophageal varix bleeding was noted again and successfully ligated by endoscopy. The disease activity of PMF was stable, and the patient underwent successful living donor liver transplantation (LDLT) on November 24, 2017.
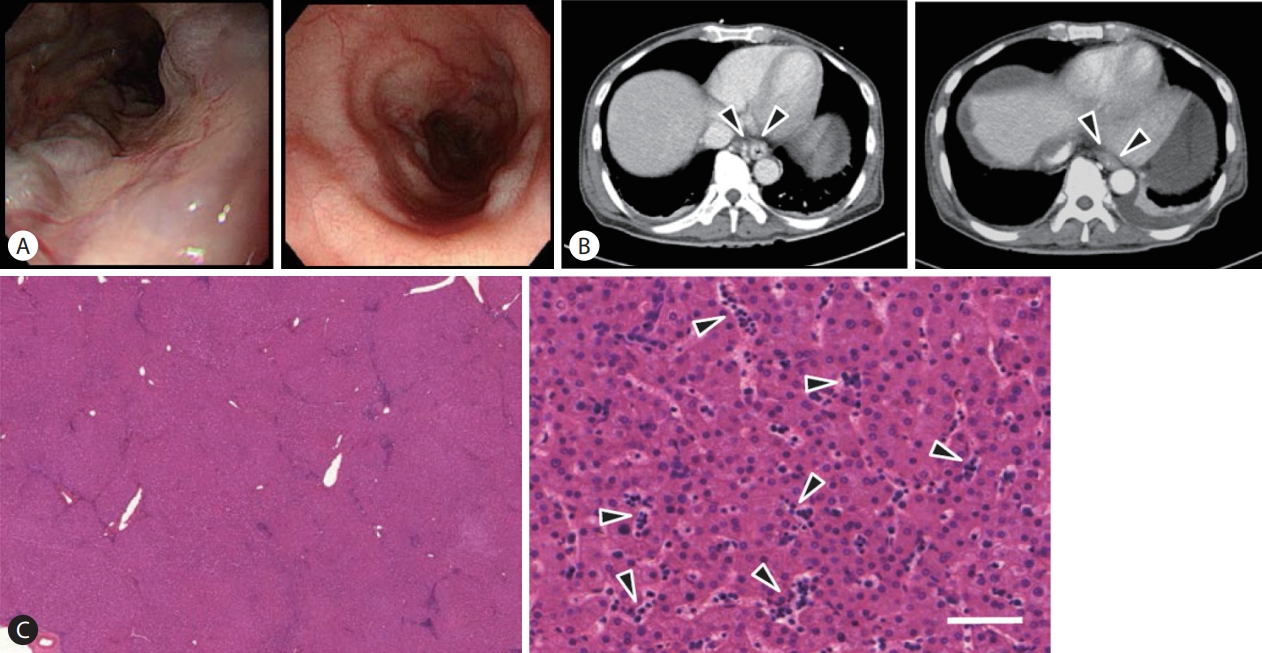
About 1 month after LDLT, in December of 2018, endoscopy revealed that varices were nearly resolved (
Fig. 1A,
B). We reviewed histologic findings of the recipient liver (
Fig. 1C). We detected diffuse myeloid metaplasia at sinusoids, verifying the previous hypothesis based on pathophysiology that extramedullary myeloid metaplasia plays an important role in PHT of MPNs. By MassonŌĆÖs trichrome staining, we found a lack of fibrotic changes in the liver parenchyma, indicating that liver parenchymal injury is not the main pathophysiological factor.
Our analysis shows that about 5% of patients with MPN who underwent abdominal CT exhibited PHT, and three among 11 patients with PHT had variceal bleeding. Interestingly, our present study showed a strong correlation between the JAK2 V617F mutation and PHT. In our study, however, ruxolitinib did not have promising results for the treatment of variceal bleeding. One patient had recurrent variceal bleeding and underwent LDLT, which dramatically decreased portal pressure.
Several previous studies have demonstrated an association between the JAK2 V617F mutation and PHT and/or portal vein thrombosis [
1,
14]. It is possible that the blood flow velocity in portal venous systems is slow, leading to prolonged interactions between blood and endothelial cells. Increased blood cells due to the clonal expansion by JAK2 V617F mutation could provoke thrombosis in the portal venous system. Hydroxyurea and JAK inhibitors are primary treatment options in MPNs. In a previous report on a patient who received ruxolitinib for PMF, PHT improved significantly, allowing discontinuation of all medications for ascites and GEVs [
9]. However, another report showed no effect of hydroxyurea and ruxolitinib on regression of PHT [
2]. Our data also showed no effect of these drugs on the size change of GEVs. The discrepancy may stem from the degree of PHT. In our study, we defined PHT as having GEVs more than 5 mm on CT, which may reflect severe PHT by MPNs.
Our study had some limitations, including the retrospective design and the use of abdominal CT to detect PHT, rather than HVPG measurement. However, owing to the inconvenience and invasiveness of the procedure, we could not recommend it for every patient.
In summary, patients with MPN can develop PHT and life-threatening events such as variceal bleeding, particularly when the patient has JAK2 V617F mutation. In one patient, LDLT resolved PHT, suggesting that liver transplantation, rather than JAK inhibitors alone, is a therapeutic candidate when MPNs are wellcontrolled. Our findings also suggest that gastrointestinal endoscopy or abdominal CT should be performed to evaluate PHT in patients with Philadelphia-negative MPNs.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



