| Clin Mol Hepatol > Volume 23(1); 2017 > Article |
|
ABSTRACT
Background/Aims
To suggest a lexicon for liver ultrasonography and to identify radiologic features indicative of benign or malignant lesions on surveillance ultrasonography.
Methods
This retrospective study included 188 nodules (benign, 101; malignant, 87) from 175 at-risk patients identified during surveillance ultrasonography for hepatocellular carcinoma. We created a lexicon for liver ultrasonography by reviewing relevant literature regarding the ultrasonographic features of hepatic lesions. Using this lexicon, two abdominal radiologists determined the presence or absence of each ultrasonographic feature for the included hepatic lesions. Independent factors associated with malignancy and interobserver agreement were determined by logistic regression analysis and kappa statistics, respectively.
Results
Larger tumor size (odds ratio [OR], 1.12; 95% confidence interval [CI], 1.06-1.183; P<0.001), multinodular confluent morphology (OR, 7.712; 95% CI, 1.053-56.465; P=0.044), thick hypoechoic rim (OR, 5.878; 95% CI, 2.681-12.888; P<0.001), and posterior acoustic enhancement (OR, 3.077; 95% CI, 1.237-7.655; P=0.016) were independently associated with malignant lesions. In a subgroup analysis of lesions <2 cm, none of the ultrasonographic features were significantly associated with malignancy or benignity. Interobserver agreement for morphology was fair (╬║=0.36), while those for rim (╬║=0.427), echogenicity (╬║=0.549), and posterior acoustic enhancement (╬║=0.543) were moderate.
Surveillance screening for hepatocellular carcinoma (HCC) has been accepted as standard care for patients with chronic liver disease who are at risk of developing HCC [1-5]. Ultrasonography (US) has become an established primary surveillance tool for the detection of HCC, given its non-invasiveness, widespread availability, acceptance by patients and physicians, and relatively low cost.
The US features of HCC, other hepatic malignancies such as metastasis or cholangiocarcinoma (CC), and benign lesions such as hemangioma have been sporadically described in literature [6-14]. However, there is a lack of uniformity in descriptive terminology for US features, which can limit its application. Therefore, the creation of a lexicon is advocated for better communication of radiological features, in order to establish standard terminology for use in daily practice and clinical research. In addition, to the best of our knowledge, no previous study has investigated the possibility of using US features for differentiating between benign and malignant lesions in a clinical setting of surveillance US for HCC.
The purposes of our study were to propose a lexicon for liver US and identify radiological features indicative of benign or malignant lesions during surveillance US.
This study was approved by our institutional review board, and informed patient consent was not required. Between January 2008 and December 2014, 8,155 patients at high risk for HCC underwent surveillance US more than once at an academic tertiary referral hospital in Seoul, Korea. Liver US and serum alpha fetoprotein (AFP) assay are routinely used in conjunction for HCC surveillance at our institution. Computed tomography (CT) or magnetic resonance imaging (MRI) is occasionally performed for the purpose of surveillance at the discretion of the clinician. Upon reviewing the medical records and imaging data of the 8,155 patients, 512 patients who were suspected for HCC during surveillance were identified. Of the 512 patients, 337 were excluded for the following reasons: suspected HCC was initially identified upon CT or MRI instead of US (n=128); the time interval between surveillance US and subsequent CT/MRI was longer than 1 month (n=198); images could not be retrieved (n=5); US image quality was too poor to allow evaluation (n=4); and hepatic lesions remained indeterminate (n=2). The final study cohort consisted of 175 patients with 188 nodules (benign, 101; malignant, 87).
Of the 101 benign lesions, while 2 were pathologically confirmed to be dysplastic nodules and focal nodular hyperplasia by biopsy (n=1, each), the remaining 99 were either not visualized (n=54) upon subsequent imaging studies or considered benign (n=45) based on the absence of changes in subsequent dynamic contrast-enhanced CT or MR images acquired during follow-up evaluations for over 2 years. Of the 87 malignant lesions, 85 were determined to be HCCs based on pathological findings or visualization of hallmark radiological findings (arterial enhancement and venous washout) upon subsequent dynamic imaging studies, while 2 were determined to be other hepatic malignancies (pathologically diagnosed CC and combined HCC-CC). Schematic representation of patient selection and diagnostic results are presented in Figure 1.
Clinical and laboratory data and pathology reports of these patients, including patient demographics, etiology of chronic liver disease, serum AFP levels, and pathological findings, were retrospectively reviewed. The reference value for serum AFP concentration used at our institution is <9 ng/mL.
Abdominal US for HCC surveillance was performed using commercially available ultrasound machines (Pro-Sound Alpha10 or Pro-Sound F75, Hitachi Aloka Medical, Tokyo, Japan; ACUSON S2000, Siemens Medical Solutions, Mountain View, CA, USA; iU22, Philips Medical Systems, Best, The Netherlands) with 5-MHz curved-array transducers. Image acquisition was performed according to our established protocol. Patients with suspected portal vein thrombosis underwent grayscale as well as Doppler imaging.
All US images were retrieved from a Picture Archiving and Communication System (Centricity, Version 2.0, GE Healthcare, Barrington, IL, USA). Two abdominal radiologists (M.S.P. and C.A., with 19 and 6 years of experience in acquisition and interpretation of abdominal US images, respectively) reviewed the literature on the US features of hepatic lesions and recorded relevant lexicons to subsequently create our own lexicon, which was applied for the evaluation of the cases included in the present study.
Based on the newly defined lexicon, two other abdominal radiologists (J.Y.L. and N.S., with 6 and 7 years of experience in abdominal US) performed blinded and independent reviews of the US images included in this study. They also evaluated the background liver parenchyma to classify it as cirrhotic or non-cirrhotic. Prior to the independent review process, they underwent training for the use of the lexicon, during which they reviewed 20 cases in consensus; these cases were not included for further analysis in our study. All US images meant for independent review were deidentified in a random order by one investigator (C.A.) and transferred to a separate workstation (Intellispace Portal 5.0, Philips, Best, The Netherlands) for blinded evaluation. Data regarding the size and number of hepatic lesions were retrieved from prospective US reports without reevaluation. Following the first independent image analysis, the interobserver agreement was evaluated, and the two reviewers drew conclusions regarding discordant results by consensus.
Comparison of variables between patients with benign and malignant hepatic lesions was performed using the Mann-Whitney U test for continuous variables and the chi-square or FisherŌĆÖs exact test for categorical variables. Correlation between US features and benignity/malignancy was determined using the chi-square or FisherŌĆÖs exact test.
The associations between US features and malignancy were determined by univariate and multivariate logistic regression analyses, and odds ratios (ORs) with 95% confidence intervals (CIs) were calculated for each of the features. Variables with alpha values <0.1 in univariate analysis were further evaluated by multivariate logistic regression analysis, where, ORs for tumor size and AFP were calculated per increments of 1 mm and 10 ng/mL, respectively.
Interobserver agreement was expressed by CohenŌĆÖs kappa or weighted-kappa coefficient (╬║). A kappa statistic value of 0.8-1.0 was considered to indicate excellent agreement; 0.6-0.79, good agreement; 0.40-0.59, moderate agreement; 0.2-0.39, fair agreement; and 0-0.19, poor agreement.15 Two-sided P-values <0.05 were considered statistically significant. All statistical analyses were performed using the SAS 9.2 software (SAS Institute Inc., Cary, NC, USA).
The demographic characteristics of the 175 patients (male, 119; female, 56; median age, 57 years; range, 27-84 years) included in this study are shown in Table 1. While 81 patients were diagnosed as having HCC or other malignancies, the remaining 94 had only benign lesions. Patients with malignant hepatic lesions were older (median age, 57 years vs. 54 years; P<0.001), more likely to be carriers of hepatitis B virus (HBV; 82.7% vs. 59.6%; P<0.001), and exhibited greater maximum lesion diameters (median diameter, 3 cm vs. 1.8 cm; P<0.001) and higher serum AFP levels (median AFP level, 10.27 ng/mL vs. 3.19 ng/mL; P<0.001) than those with benign lesions. There were no significant differences between the two patient groups in terms of sex (P =0.255), background liver (P =0.302), or number of suspicious lesions identified on surveillance US (P =0.78).
The schematic drawing and description of our lexicon for liver US are presented in Figure 2.
The lexicon has four categories:
1) Morphology ŌĆö nodular with indistinct margin, simple nodular, multinodular confluent, or infiltrative
2) Rim ŌĆö none, hyperechoic, thin (<2 mm) hypoechoic, or thick (Ōēź2 mm) hypoechoic (Figs. 3 and 4)
3) Echogenicity ŌĆö homogeneously hyperechoic, homogeneously isoechoic, homogeneously hypoechoic, heterogeneous, or mosaic appearance
4) Posterior acoustic enhancement ŌĆö absent, present, or non-assessable (in case of lesions located in the posterior subcapsular portions of the liver)
The results of image analysis are presented in Table 2. Benign hepatic lesions were more likely to exhibit no rim (P<0.001) and homogeneous hyperechogenicity (P<0.001) than malignant lesions, while the latter were more likely to exhibit multinodular confluent morphology (P =0.02), thick hypoechoic rim (P<0.001), heterogeneous echogenicity (P <0.001), mosaic appearance (P =0.04), and posterior acoustic enhancement (P<0.001) than benign lesions. Interobserver agreement for morphology (╬║=0.36) was fair, while those for rim (╬║=0.427), echogenicity (╬║=0.549), and posterior acoustic enhancement (╬║=0.543) were moderate.
The results of univariate and multivariate logistic regression analyses (Table 3) revealed larger tumor size (OR, 1.12; 95% CI, 1.06-1.183; P<0.001), multinodular confluent morphology (OR, 7.712; 95% CI, 1.053-56.465; P =0.044), thick hypoechoic rim (OR, 5.878; 95% CI, 2.681-12.888; P<0.001), and posterior acoustic enhancement (OR, 3.077; 95% CI, 1.237-7.655; P =0.016) to be independent factors associated with malignant hepatic lesions. None of the US features were significantly associated with benign lesions.
Prevalence of malignancy according to tumor size is presented in Table 4. Of the 188 evaluated lesions, 14 (7.4%) were subcentimeter (<1 cm) lesions, 62 (33%) were 1-2 cm in size, 57 (30.3%) were 2-3 cm, and 55 (29.3%) were 3 cm or larger. None (0%) of the subcentimeter lesions, 14 (22.6%) of the 1-2 cm lesions, 30 (52.6%) of the 2-3 cm lesions, and 43 (78.2%) of the lesions Ōēź3 cm were malignant.
The results of subgroup analysis of lesions <2 cm revealed that none of the US features were significantly associated with malignancy or benignity (Table 5). Furthermore, US features favoring malignancy were rarely observed in small lesions; among the 14 small (<2 cm) malignant lesions, thick hypoechoic rim, heterogeneous echogenicity, mosaic appearance, and posterior acoustic enhancement were observed in none or only a couple of cases (Table 5). Logistic regression analysis could not be performed because the frequencies of potentially significant US features were too low.
In the present study, size and three morphological features including multinodular confluent morphology, thick hypoechoic rim, and posterior acoustic enhancement were found to be significantly associated with malignancy. Multinodular confluent morphology, thick hypoechoic rim, and posterior acoustic enhancement were reported as morphological features suggestive of malignancy over two decades ago [8,10,12,14]. In spite of the recent technological developments in US, characteristic features suggestive of malignancy have remained unchanged. However, in our study, these features were mostly observed in large lesions. In addition, in case of hepatic lesions <2 cm in size, none of the US features exhibited significant association with benignity or malignancy.
All the international guidelines clearly state that US is a surveillance tool, not diagnostic [1-5]. According to the current guidelines, short-term follow-up is recommended for a hepatic lesion smaller than 1 cm found on surveillance US, while for a hepatic lesion larger than 1 cm, dynamic contrast-enhanced CT or MRI is recommended as a recall policy irrespective of US features. Our results support the recommendation by the current guidelines; in our study, all of the subcentimeter nodules were benign, and the potential for malignancy increases by more than 20% with the increase in the size of lesions beyond 1 cm, with any US feature unable to differentiate between small HCC and benign lesion.
Previous studies reporting that hypoechoic rim is suggestive of malignancy have not taken the thickness of the rim into account [9,10,12]. To reflect the evolution of technology, we divided the hypoechoic rim category into two subcategories ŌĆö thin and thick. In our study, thick hypoechoic rim was significantly associated with malignancy, while thin hypoechoic rim exhibited no significant association. Thin hypoechoic rims observed around benign lesions are more likely to be pseudo-rims, i.e., Mach bands rather than true rims, created because of an optical effect at margins between areas of different echogenicities [16]. In contrast, hyperechoic rim with partially hypoechoic internal pattern has been reported as being specific for hepatic hemangioma [11,13]. In our study, 5 of 6 lesions with hyperechoic rims were benign, which suggests that hyperechoic rim might be indicative of benign lesions; however, the statistical significance of this association could not be established in our study, possibly because of the low frequency of occurrence of hyperechoic rims.
A major limitation of this study is that we retrospectively reviewed US images acquired by a heterogeneous group of US operators, including inexperienced ones. Therefore, the results of our study might not be relevant when prospectively applied in different settings. Another limitation could be that the difference of size distribution between benign and malignant lesions, which could affect the results. Our results showed that none of the US features was found to be significantly associated with benignity or malignancy in case of small (<2 cm) hepatic lesions. Among the 76 small nodules, only 14 (18.5%) nodules were malignant.
In conclusion, for hepatic lesions larger than 2 cm, some US features might be suggestive of malignancy. We proposed a lexicon that may be useful for surveillance US.
ACKNOWLEDGMENTS
This study was supported by a grant from ŌĆ£The Research Supporting Program of the Korean Association for the Study of the LiverŌĆØ.
The authors would like to thank Dong-Su Jang, MFA (Medical Illustrator, Medical Research Support Section, Yonsei University College of Medicine, Seoul, Korea) for his help with the illustrations.
FOOTNOTES
Figure┬Ā1.
Flow diagram of the patient selection process and diagnostic results. HCC, hepatocellular carcinoma; CT, computed tomography; MRI, magnetic resonance imaging; US, ultrasonography; DN, dysplastic nodule; FNH, focal nodular hyperplasia; CC, cholangiocarcinoma; cHCC-CC, combined HCC-CC.
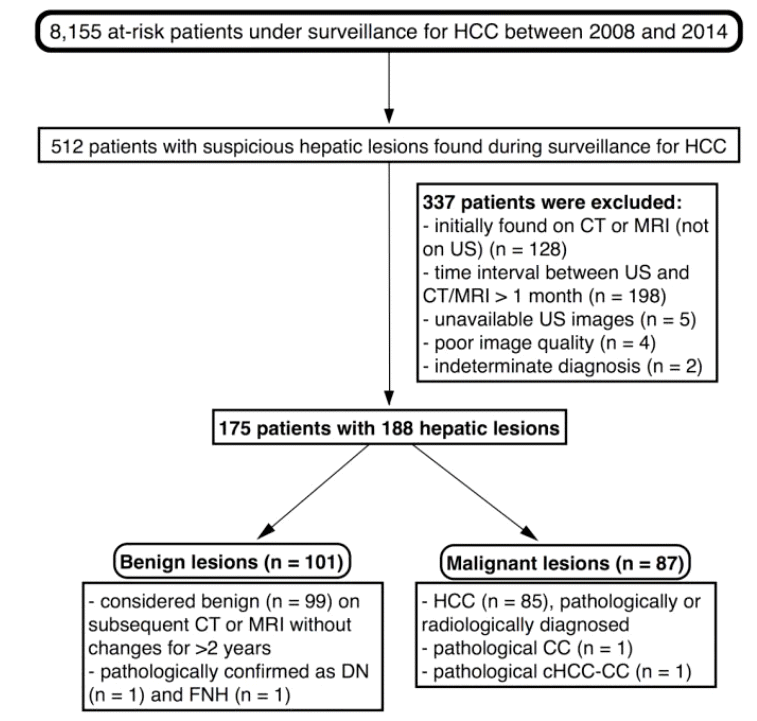
Figure┬Ā3.
Thin and thick hypoechoic rims. (A) A 41-year-old man with chronic hepatitis B. A 2.3-cm hyperechoic nodule in S4 of the liver was detected on surveillance ultrasonography. The nodule had a sharply demarcated border, causing a thin hypoechoic halo appearance (arrow). Additionally, acoustic enhancement was observed posterior to the nodule. Upon magnetic resonance imaging (MRI), the nodule was diagnosed as a hemangioma based upon typical imaging features. (B) A 27-year-old man with B-viral liver cirrhosis. A 1-cm hyperechoic nodule was detected in S7 of the liver, with a barely recognizable thin hypoechoic halo. The nodule exhibited typical imaging features of hemangioma on computed tomography (CT). (C) A 56-year-old man with B-viral liver cirrhosis. A 2.1-cm nodule with a relatively thick hypoechoic rim was seen in S8 of the liver. Additionally, posterior acoustic enhancement was observed. The nodule was diagnosed as hepatocellular carcinoma based on CT and MRI findings.

Figure┬Ā4.
Hyperechoic rim suggestive of benignity. (A) A 43-year-old man with B-viral liver cirrhosis. A 1.3-cm nodule in S5 exhibited a distinct hyperechoic rim with less echogenic portions at the center. The most likely diagnosis of this nodule based on magnetic resonance imaging findings was dysplastic nodule, and it exhibited no changes in size or characteristics for over 2 years. (B) A 43-year-old man with chronic hepatitis B. A 2.1-cm hyperechoic lesion exhibited a relatively less echogenic area at the center. This nodule exhibited typical imaging features of hemangioma and showed no growth for over 2 years.
Table┬Ā1.
Baseline characteristics of patients
Table┬Ā2.
Interobserver agreement and frequency of ultrasonographic features in benign and malignant hepatic lesions
| Benign (n=101) | Malignant (n=87) | Total (n=188) | P-value | |
|---|---|---|---|---|
| Morphology (╬║=0.36)* | ||||
| ŌĆāNodular with indistinct margin | 36 (35.6) | 37 (42.5) | 73 (38.8) | 0.999 |
| ŌĆāSimple nodular | 63 (62.4) | 45 (51.7) | 108 (57.4) | 0.183 |
| ŌĆāMultinodular confluent | 0 (0) | 5 (5.7) | 5 (2.7) | 0.02 |
| ŌĆāInfiltrative | 2 (2) | 0 (0) | 2 (1.1) | 0.5 |
| Rim (╬║=0.427)* | ||||
| ŌĆāNone | 71 (70.3) | 39 (44.8) | 110 (58.5) | <0.001 |
| ŌĆāHyperechoic | 5 (5) | 1 (1.1) | 6 (3.2) | 0.219 |
| ŌĆāThin hypoechoic | 15 (14.9) | 13 (14.9) | 28 (14.9) | 0.999 |
| ŌĆāThick hypoechoic | 10 (9.9) | 34 (39.1) | 44 (23.4) | <0.001 |
| Echogenicity (╬║=0.549)* | ||||
| ŌĆāHomogeneously hyperechoic | 47 (46.5) | 12 (13.8) | 59 (31.4) | <0.001 |
| ŌĆāHomogeneously isoechoic | 9 (8.9) | 13 (14.9) | 22 (11.7) | 0.999 |
| ŌĆāHomogeneously hypoechoic | 28 (27.7) | 20 (23) | 48 (25.5) | 0.505 |
| ŌĆāHeterogeneous | 17 (16.8) | 38 (43.7) | 55 (29.3) | <0.001 |
| ŌĆāMosaic appearance | 0 (0) | 4 (4.6) | 4 (2.1) | 0.04 |
| Posterior acoustic enhancement (╬║=0.543)* | ||||
| ŌĆāAbsent | 72 (96) | 40 (65.6) | 112 (82.4) | |
| ŌĆāPresent | 3 (4) | 21 (34.4) | 24 (17.6) | <0.001 |
| ŌĆāNon-assessable | 26 | 26 | 52 |
Table┬Ā3.
Logistic regression analysis of ultrasonographic (US) features associated with benign and malignant hepatic lesions
| US feature |
Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Size* | 1.1 | 1.063-1.139 | <0.001 | 1.12 | 1.060-1.183 | <0.001 |
| Morphology | ||||||
| ŌĆāNodular with indistinct margin | Reference | |||||
| ŌĆāSimple nodular | 1.46 | 0.898-2.374 | 0.127 | |||
| ŌĆāMultinodular confluent | 13.265 | 2.822-62.35 | 0.001 | 7.712 | 1.053-56.465 | 0.044 |
| ŌĆāInfiltrative | 1.561 | 0.315-7.722 | 0.585 | |||
| Rim | ||||||
| ŌĆāNone | Reference | |||||
| ŌĆāHyperechoic | 0.324 | 0.065-1.601 | 0.167 | |||
| ŌĆāThin hypoechoic | 1.986 | 0.961-4.107 | 0.064 | 1.552 | 0.476-5.053 | 0.466 |
| ŌĆāThick hypoechoic | 5.976 | 2.735-13.058 | <0.001 | 5.878 | 2.681-12.888 | <0.001 |
| Echogenicity | ||||||
| ŌĆāHomogeneously hyperechoic | Reference | |||||
| ŌĆāHomogeneously isoechoic | 0.58 | 0.241-1.398 | 0.225 | |||
| ŌĆāHomogeneously hypoechoic | 0.413 | 0.172-0.991 | 0.048 | 1.236 | 0.343-4.454 | 0.746 |
| ŌĆāHeterogeneous | 0.807 | 0.362-1.798 | 0.599 | |||
| ŌĆāMosaic appearance | 0.741 | 0.123-4.461 | 0.743 | |||
| Posterior acoustic enhancement | ||||||
| ŌĆāAbsent or Non-assessable | Reference | |||||
| ŌĆāPresent | 5.353 | 2.352-12.184 | <0.001 | 3.077 | 1.237-7.655 | 0.016 |
Table┬Ā4.
Prevalence of hepatic malignancy according to tumor size
Table┬Ā5.
Distribution of ultrasonographic (US) features among hepatic lesions <2 cm in size
| Benign (n=62) | Malignant (n=14) | Total (n=76) | |
|---|---|---|---|
| Morphology | |||
| ŌĆāNodular with indistinct margin | 17 (27.4) | 5 (35.7) | 22 (28.9) |
| ŌĆāSimple nodular | 45 (72.6) | 8 (57.1) | 53 (69.7) |
| ŌĆāMultinodular confluent* | 0 (0) | 1 (7.1) | 1 (1.3) |
| ŌĆāInfiltrative | 0 (0) | 0 (0) | 0 (0) |
| Rim | |||
| ŌĆāNone | 45 (72.6) | 10 (71.4) | 55 (72.4) |
| ŌĆāHyperechoic | 3 (4.8) | 0 (0) | 3 (3.9) |
| ŌĆāThin hypoechoic | 7 (11.3) | 4 (28.6) | 11 (14.5) |
| ŌĆāThick hypoechoic* | 7 (11.3) | 0 (0) | 7 (9.2) |
| Echogenicity | |||
| ŌĆāHomogeneous hyperechoic | 36 (58.1) | 6 (42.9) | 42 (55.3) |
| ŌĆāHomogeneous isoechoic | 2 (3.2) | 1 (7.1) | 3 (3.9) |
| ŌĆāHomogeneous hypoechoic | 18 (29) | 6 (42.9) | 24 (31.6) |
| ŌĆāHeterogeneous | 6 (9.7) | 1 (7.1) | 7 (9.2) |
| ŌĆāMosaic appearance | 0 (0) | 0 (0) | 0 (0) |
| Posterior acoustic enhancement | |||
| ŌĆāAbsent | 50 (98) | 10 (83.3) | 60 (95.2) |
| ŌĆāPresent* | 1 (2) | 2 (16.7) | 3 (4.8) |
| ŌĆāNon-assessable | 11 | 2 | 13 |
REFERENCES
1. Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int 2010;4:439-474.



2. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-1022.



3. European Organisation For Research; European Association For The Study Of The Liver. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-943.


4. Kudo M, Matsui O, Izumi N, Iijima H, Kadoya M, Imai Y, et al. Surveillance and diagnostic algorithm for hepatocellular carcinoma proposed by the Liver Cancer Study Group of Japan: 2014 update. Oncology 2014;87(Suppl 1):7-21.

5. Lee JM, Park JW, Choi BI. 2014 KLCSG-NCC Korea Practice Guidelines for the management of hepatocellular carcinoma: HCC diagnostic algorithm. Dig Dis 2014;32:764-777.


6. Shinagawa T, Ohto M, Kimura K, Tsunetomi S, Morita M, Saisho H, et al. Diagnosis and clinical features of small hepatocellular carcinoma with emphasis on the utility of real-time ultrasonography. Gastroenterology 1984;86:495-502.


7. Sheu JC, Chen DS, Sung JL, Chuang CN, Yang PM, Lin JT, et al. Hepatocellular carcinoma: US evolution in the early stage. Radiology 1985;155:463-467.


8. Choi BI, Kim CW, Han MC, Kim CY, Lee HS, Kim ST, et al. Sonographic characteristics of small hepatocellular carcinoma. Gastrointest Radiol 1989;14:255-261.


9. Wernecke K, Henke L, Vassallo P, von Bassewitz DB, Diederich S, Peters PE, et al. Pathologic explanation for hypoechoic halo seen on sonograms of malignant liver tumors: an in vitro correlative study. AJR. Am J Roentgenol 1992;159:1011-1016.

10. Wernecke K, Vassallo P, Bick U, Diederich S, Peters PE. The distinction between benign and malignant liver tumors on sonography: value of a hypoechoic halo. AJR Am J Roentgenol 1992;159:1005-1009.


11. Moody AR, Wilson SR. Atypical hepatic hemangioma: a suggestive sonographic morphology. Radiology 1993;188:413-417.


12. Shibata T, Sakahara H, Kawakami S, Konishi J. Sonographic characteristics of recurrent hepatocellular carcinoma. Eur Radiol 1996;6:443-447.


13. Kim KW, Kim TK, Han JK, Kim AY, Lee HJ, Park SH, et al. Hepatic hemangiomas: spectrum of US appearances on gray-scale, power Doppler, and contrast-enhanced US. Korean J Radiol 2000;1:191-197.



14. Minami Y, Kudo M. Hepatic malignancies: Correlation between sonographic findings and pathological features. World J Radiol 2010;2:249.







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




