| Clin Mol Hepatol > Volume 23(4); 2017 > Article |
|
See the commentary-article "Lamivudine: fading into the mists of time" on page 314.
ABSTRACT
Background/Aims
Long-term data on antiviral therapy in Korean patients with hepatitis B e antigen (HBeAg)-negative chronic hepatitis B (CHB) are limited. This study evaluated the efficacy and safety of entecavir (ETV) and lamivudine (LAM) over 240 weeks.
Methods
Treatment-naive patients with HBeAg-negative CHB were randomized to receive ETV 0.5 mg/day or LAM 100 mg/day during the 96 week double-blind phase, followed by open-label treatment through week 240. The primary endpoint was the proportion of patients with virologic response (VR; hepatitis B virus [HBV] DNA<300 copies/mL) at week 24. Secondary objectives included alanine aminotransferase (ALT) normalization and emergence of ETV resistance (week 96), VR and log reduction in HBV DNA levels (week 240), and safety evaluation.
Results
In total, 120 patients (>16 years old) were included (ETV, n=56; LAM, n=64). Baseline characteristics were comparable between the two groups. A significantly higher proportion of ETV-treated patients achieved VR compared to LAM at week 24 (92.9% vs. 67.2%, P=0.0006), week 96 (94.6% vs. 48.4%, P<0.0001), and week 240 (95.0% vs. 47.6%, P<0.0001). At week 96, ALT normalization was observed in 87.5% and 51.6% of ETV and LAM patients, respectively (P<0.0001). Virologic breakthrough occurred in one patient (1.8%) receiving ETV and 26 patients (42.6%) receiving LAM (P<0.0001) up to week 96. Emergence of resistance to ETV was not detected. The incidence of serious adverse events was low and unrelated to the study medications.
Chronic hepatitis B (CHB) is a significant health concern in Korea and a leading cause of cirrhosis and hepatocellular carcinoma. 1,2 Although the incidence of CHB in Korea has reduced in response to the introduction of vaccination programmes, the country is still classified as an intermediate endemic area, with hepatitis B virus (HBV) prevalence rates ranging from 2 to 7% [1,2].
Mutations in the precore or core promoter region of HBV may result in hepatitis B e antigen (HBeAg)-negative CHB [3]. Patients who are HBeAg-negative are difficult to cure as disease progression is rapid and aggressive in this population [3,4]; the annual rate of progression to cirrhosis in these patients is 8ŌĆō10%, while that in HBeAg-positive patients is 2ŌĆō5% [3]. Persistently elevated alanine aminotransferase (ALT) levels are observed in 30ŌĆō40% of CHB patients who are HBeAg-negative, while the remainder experience alanine aminotransferase (ALT) flares that tend to be refractory to antiviral treatment (interferon [IFN]-alpha), with sustained remission observed in only 6ŌĆō15% of the latter [5]. Furthermore, poor long-term prognosis, higher rates of immune reactivation and the lower likelihood of spontaneous HBsAg seroclearance are critical challenges in the management of these patients [3,6,7].
International guidelines recommend either finite treatment with pegylated IFN (PEG-IFN) for 48 weeks, or long-term nucleos(t)ide analogues (NUC) treatment in CHB patients who are HBeAg-negative [8,9]. However, treatment with PEG-IFN in HBeAg-negative patients has been shown to yield low virologic outcomes, especially in patients with HBV genotype C due to a high number of basic core and precore mutations [10-12]. As genotype C is widely prevalent in Korean CHB patients, this observation implies that treatment with PEG-IFN may not be optimal for the population in this region [13,14]. Furthermore, the frequency of severe adverse events (AEs) contributes to the early discontinuation of treatment. Longterm NUC treatment, on the other hand, has provided a convenient, efficacious and safe option for these patients [15]. Entecavir (ETV) and tenofovir disoproxil fumarate (TDF) are recommended as first-line therapeutic options by international guidelines and are associated with a minimal risk for development of resistance [8,9,16]. Furthermore, ETV and TDF were recently added to the latest edition (2015) of the WHO Model List of Essential Medicines as the only treatment options for HBV, and ETV is the recommended option in children [17]. Although lamivudine (LAM) is effective in the treatment of HBeAg-negative patients, long-term outcomes are limited by the emergence of resistance [5].
Studies have demonstrated that ETV therapy has superior virologic, biochemical and serological outcomes compared with LAM in both HBeAg-positive and HBeAg-negative patients [18,19]. Recently, a retrospective analysis of 5,374 Korean patients demonstrated that, compared to LAM, ETV is associated with a significantly lower risk of death or liver transplantation [20]. Long-term ETV treatment in HBeAg-negative patients from other Asian countries has also shown excellent virologic outcomes [19,21,22]. However, to date, only short-term trials have reported the efficacy of ETV treatment in HBeAg-negative Korean patients and data on long-term treatment in HBeAg-negative patients are limited [19,23]. Our trial, therefore, was conducted in treatment-naive Korean patients with HBeAg-negative CHB to evaluate the efficacy and safety of ETV and LAM following 240 weeks of treatment.
This Phase IV randomized trial was conducted in 16 hospitals across South Korea from 30 January 2007 to 4 March 2013, in accordance with the International Conference of Harmonisation Guidelines for Ethical Conduct and approved by the Clinical Trials Review committee of each institution (this study has been registered at ClinicalTrials. gov under registration number NCT00393484). The study was double-blinded for 96 weeks followed by open-label treatment until Week 240. Randomized block design stratification was employed, wherein a unique identification code was assigned to each patient during registration. Patients, investigators and the sponsorŌĆÖs staff were blinded during allocation of the study medication and during the 96-week treatment period. Patients who were willing to participate in the open-label phase of the study were unblinded on an individual basis to determine further management. As this study was planned from 2005 when LAM was the standard of care in chronic hepatitis B treatment in Korea, no data on the use of ETV in Korean patients with HBeAg-negative chronic HBV infection had been released at the time. HBeAg-negative disease represents an important segment of the Korean adult population with chronic HBV infection, thus additional information was needed regarding the efficacy of ETV with long-term use in these HBeAg negative patients.
Adult (>16 years old) Korean patients with CHB who were HBeAg-negative (i.e. HBeAg-negative and anti-HBe-positive) and hepatitis B surface antigen (HBsAg)-positive for Ōēź6 months were included in the study. These patients were NUC-naive with compensated liver function and HBV DNA levels Ōēź105 copies/mL (Ōēź1 occurrence and more than 4 weeks prior to screening). Additional inclusion criteria included elevated ALT 1.3ŌĆō10 ├Ś upper limit of normal (ULN; Ōēź1 occurrence at the time of screening and 4 weeks prior to screening), international normalised ratio Ōēż1.5, serum albumin levels Ōēź3 g/dL (Ōēź30 g/L) and serum bilirubin levels Ōēż2.5 mg/dL (Ōēż42.75 ╬╝mol/L).
Patients treated with IFNs within 24 weeks of randomization were excluded from the study. Women who were pregnant or breastfeeding, those of childbearing potential, and those who were unable to use contraceptives during the study period and up to 8 weeks post-study completion; patients with pre-existing diseases such as variceal bleeding, hepatic encephalopathy, ascites, hepatorenal syndrome, human immunodeficiency virus, co-infection with hepatitis C or D; patients with hepatocellular carcinoma; patients awaiting liver transplantation; and patients with serum alpha fetoprotein >100 ng/mL were not included. Concurrent medications that could lead to liver or kidney toxicity were contraindicated throughout the study.
Written informed consent was obtained from all patients who participated in the study. These patients were randomly assigned to receive ETV (0.5 mg/day) or LAM (100 mg/day). The first medication dose was administered within 72 hours of randomization. During the double-blind period, patients in the ETV group received one ETV tablet and one tablet of LAM placebo, while patients in the LAM group received one LAM tablet and one tablet of ETV placebo. Patients were instructed to take their medication at the same time every day, typically 2 hours before or after a meal.
The primary efficacy endpoint was the proportion of patients who achieved virologic response (VR; HBV DNA <300 copies/mL) at week 24 of treatment with ETV and LAM. Secondary endpoints included the proportion of patients with VR at weeks 96 and 240; mean log reduction in HBV DNA levels through to week 240; serum ALT levels until week 96 and emergence of resistance to ETV. Development of resistance was defined as manifestation of amino acid substitutions conferring ETV resistance in patients who met the criterion for virologic rebound (>1 log10 increase in HBV DNA from nadir on blinded treatment determined by two sequential HBV DNA measurements by polymerase chain reaction [PCR] assay, or last on-treatment measurement). Since genotypic resistance to LAM is well documented based on previous clinical trial data, we did not include this evaluation in our study [8,9]. Virologic breakthrough was defined according to the American Association for the Study of Liver Diseases (AASLD) guidelines as increase in serum HBV DNA by Ōēź1 log10 (10-fold) above nadir after achieving virologic response, during continued treatment. The frequency of AEs, serious adverse events (SAEs), discontinuations due to AEs and laboratory abnormalities were reported as safety outcomes.
HBV DNA was analysed at weeks 24, 48, 96, 192 and 240 using the Roche COBAS Amplicor PCR assay (Roche Diagnostics Systems Inc., Pleasanton, CA, USA) until the year 2010, followed by COBAS Taqman® HBV Real-Time PCR assay (Roche Molecular Systems Inc., Branchburg, NJ, USA) from 2011 using the same endpoint for VR (HBV DNA <300 copies/mL). Other laboratory tests were performed at Weeks 24, 48 and 96 in accordance with standardised procedures in a centralised location.
Therapy was discontinued if one of the following criteria were met: (1) cancellation of written consent by patient; (2) upon investigatorsŌĆÖ judgement that continuing medication was not appropriate for patients due to clinical adverse reaction, abnormal lab test results or concurrent disease; (3) pregnancy; or (4) confinement of patients due to physical (such as infectious disease) or mental treatments.
A two-stage evaluation, using a non-completer equals failure analysis for the efficacy of ETV compared with LAM was conducted. The first stage test was carried out to establish the non-inferiority of ETV over LAM, while the second stage analysis was a test for treatment superiority. Since the superiority test was carried out only after establishing non-inferiority, the significance levels were not adjusted for the first stage of testing. Non-inferiority was established when the lower limit of the 90% confidence interval (CI) was above ŌĆō10%, while superiority was established when the 90% CI was above 0% at week 24.
A sample size of 60 patients per group was considered sufficient to yield 83% power to demonstrate non-inferiority for the difference in the primary endpoint, as well as to establish the superiority of ETV over LAM. Categorical data are presented as frequency and percentages, and statistical significance was calculated using the PearsonŌĆÖs chi-square test or FisherŌĆÖs exact test. Continuous data are presented as mean┬▒standard deviation and/or median values. Statistical significance was determined using the unpaired t-test, paired t-test, Wilcoxon rank-sum test, or Wilcoxon signed-rank test depending on the Gaussian satisfaction criteria. All analytical data were set at a double-sided significance level of 0.05.
A total of 200 patients were screened, of whom 122 met the inclusion criteria and were randomized to receive ETV 0.5 mg/day (n=57) or LAM 100 mg/day (n=65). Patient disposition until week 240 is presented in Figure 1. The most common reason for study discontinuation in the ETV group was withdrawal of consent, while in the LAM group, treatment failure/lack of efficacy was the most common reason cited (Fig. 1). Demographic and baseline characteristics were comparable between the two groups (Table 1).
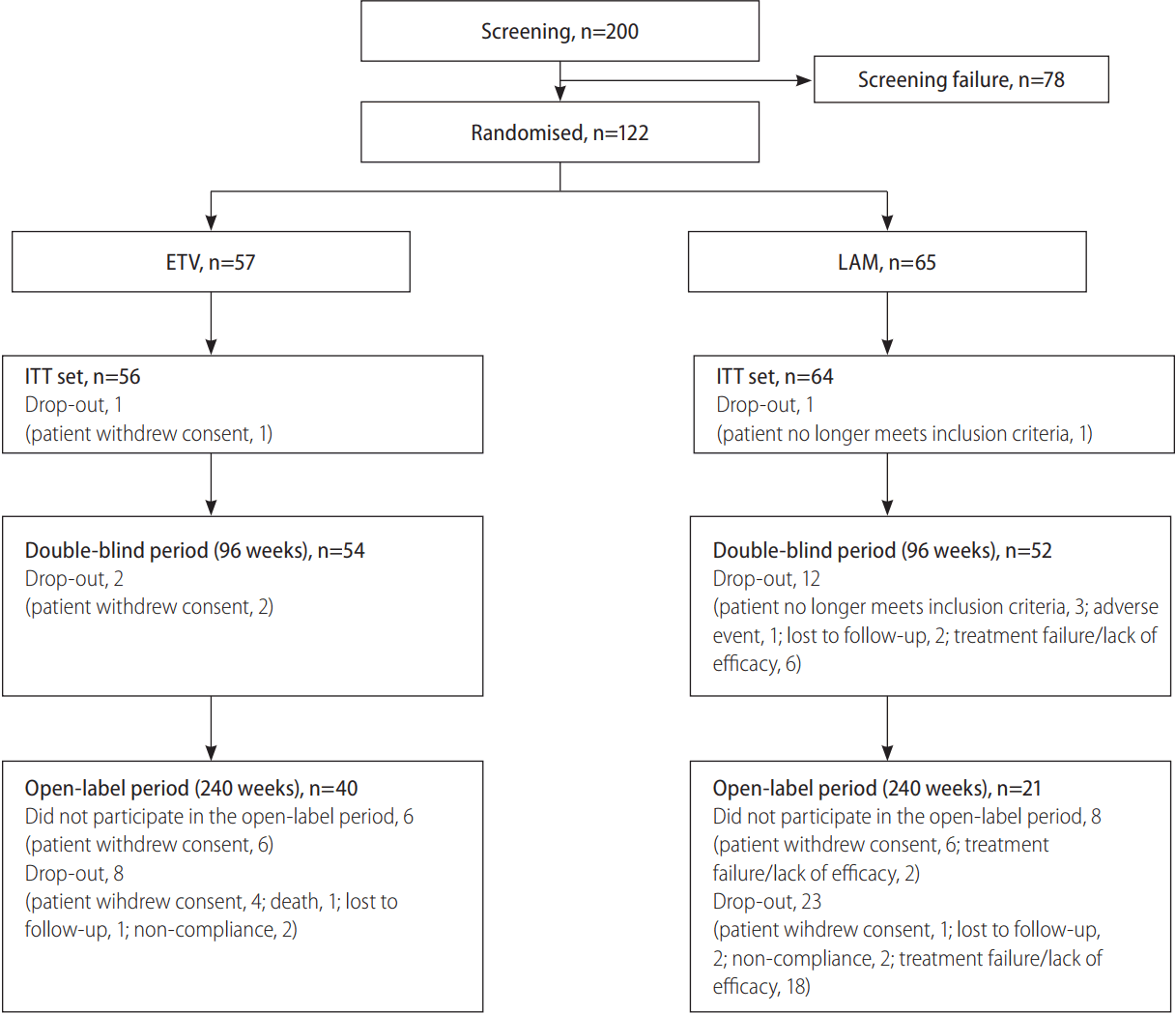
A significantly higher proportion of patients treated with ETV achieved VR compared with those receiving LAM at week 24 (92.9% vs. 67.2%; P=0.0006) (Fig. 2) and week 96 (94.6% vs. 48.4%; P<0.0001). After 240 weeks of treatment, VR was observed in 95.0% of ETV-treated patients and 47.6% of LAMtreated patients (P<0.0001) (Fig. 2).
The mean log reduction in HBV DNA throughout the study duration is shown in Figure 3. Patients treated with ETV had a significantly higher log reduction in HBV DNA (ŌĆō3.6┬▒0.8 log10 copies/mL) compared with those receiving LAM (ŌĆō2.5┬▒1.5 log10 copies/mL, P<0.0001) at week 240.
ALT normalization was observed in a higher proportion of ETVtreated patients than LAM-treated patients in the double-blind period: week 24 (ETV, 43 [76.8%] patients vs. LAM, 37 [57.8%] patients; P=0.03) and week 96 (ETV, 49 [87.5%] patients vs. LAM, 33 [51.6%] patients; P<0.0001). Virologic breakthrough occurred in one (1.8%) ETV-treated patient and 26 (42.6%) LAMtreated patients (P<0.0001) at week 96 (Table 2). No emergent resistance was detected in this one ETV-treated patient.
Overall, both treatments were well tolerated. AEs reported in both groups up to week 240 are presented in Table 3. Serious AEs (SAEs) were reported in seven ETV-treated patients and 17 LAMtreated patients; however, no event was found to be related to the study medications. The most common SAEs in the ETV group were classified under ŌĆøinjury, poisoning and procedural complications,ŌĆÖ while ŌĆøneoplasmsŌĆÖ were the most frequent SAEs in the LAM group (Table 3). Death, reported for one patient in the ETV group, occurred due to subarachnoid haemorrhage in a road accident and was unrelated to the study medication. One patient in the LAM group discontinued due to an SAE (cellulitis) that was not considered likely to be related to LAM treatment.
Laboratory abnormalities reported until week 96 have been summarized in Table 3. Grade 3/4 increase in ALT and aspartate aminotransferase (AST) was observed in six (9.7%) and three (4.8%) LAM-treated patients, respectively. None of the patients in the ETV-treated cohort experienced grade 3/4 abnormalities in ALT or AST. At Week 96, eight (12.9%) LAM-treated patients reported ALT and AST >2 ├Ś the baseline levels. Additionally, ALT flare (ALT >2 ├Ś baseline and >10 ├Ś ULN) was noted in one patient.
This randomized controlled trial in Korean patients with treatment-naive HBeAg-negative CHB showed that long-term ETV treatment was associated with significantly greater virologic outcomes and biochemical responses than LAM. During the double-blind and open-label study periods, a higher proportion of patients in the ETV group achieved VR at weeks 24, 96 and 240 than in the LAM group. Importantly, potent viral suppression with ETV was accompanied by a decreased risk of developing resistance to antiviral treatment.
Routine monitoring of HBV DNA levels is important in HBeAg-negative patients due to the high propensity of early relapse [24]. In this study, HBV DNA levels were monitored at 12-week intervals through to week 240, and with ETV treatment, resulting in a significantly higher mean reduction in HBV DNA than with LAM treatment (95.0% vs. 47.6%). These results corroborate previous observations of ETV-induced viral suppression following long-term trials in Asian patients and shortterm trials in Korean patients [4,25]. The mean VR rate after 5 years of ETV treatment was 98% in 1,126 treatment-naive Asian patients, 76% of whom were HBeAg-negative [4]. Another trial indicated VR in 96.6% of HBeAg-negative Korean patients receiving ETV for at least 12 months [25]. In our study, a higher proportion of patients treated with ETV experienced ALT normalization compared to those receiving LAM. Virologic breakthrough was observed in 26 LAM-treated patients. In comparison, only one ETV-treated patient experienced virologic breakthrough, which was not related to the development of antiviral resistance. This patient was a 51 year-old Korean man, with a body mass index of 25.97 kg/m2. At baseline, he was diagnosed with liver cirrhosis and renal cysts, and regularly consumed alcohol and tobacco. Before this ETV-treated patient withdrew consent from the open-label study period, no ALT flares were reported during treatment. After virologic breakthrough (detected at 48 weeks), HBV-DNA levels were below assay detection levels between weeks 60 to 192, inclusive. Since this patient dropped out during the open-label study period, it was difficult to say whether continued ETV treatment had any improvement on his condition. However, ETV-treated patients showing virologic breakthrough but not ETV resistance have been reported before [18,19].
HBeAg-negative patients require long-term treatment even if HBV DNA levels remain undetectable during sustained therapy in order to prevent relapse, disease progression and development of fatal complications. In Asian HBeAg-negative patients, ETV cessation following Ōēź2 years of treatment, as per the Asian Pacific Association for the Study of the Liver criteria, led to virologic relapse in 74.2% and 91.4% of patients at weeks 24 and 48, respectively [26]. Shouval et al. indicated that the majority of HBeAg-negative patients who were complete responders to NUC therapy in the first year relapsed following treatment discontinuation [27]. Although there is no consensus on the timing of treatment discontinuation in HBeAg-negative patients, these studies emphasize the need for continual antiviral therapy. Development of antiviral resistance is the most important concern of long-term treatment; therefore, international guidelines recommend ETV or TDF as first-line therapies due to their high resistance barrier [8,9,16]. Notably, in our study, resistance to ETV was not detected in any patient up to 240 weeks of treatment. Our data confirm previous reports of low ETV resistance rates (1.2%) following treatment for up to 6 years [28]. Although not evaluated in this study design, the development of resistance to LAM is well established and is the cause of suboptimal treatment outcomes. Up to 80% of LAM-treated patients are known to develop resistance after 5 years [29,30]. The emergence of LAM-resistant mutations also coincides with the reappearance of HBV DNA and ALT elevation [31].
It is well documented that safety and tolerability of treatment regimens are associated with better patient compliance, thus resulting in better virologic outcomes. Both ETV and LAM were well tolerated with a low incidence of SAEs that were not related to the study medications. However, a considerable number of patients in the LAM group withdrew due to treatment failure. These observations confirm the established barriers of long-term LAM therapy. Conversely, the rate of discontinuation in the ETV group during the double-blind period was very low, as also observed in previous studies that have shown an average 3-year discontinuation rate of 1% [4].
We acknowledge that the results of our study are limited by the small sample size and high drop-out rate in the LAM group. Nevertheless, this study demonstrated the efficacy and safety of ETV for up to 240 weeks of treatment in Korean HBeAg-negative patients with CHB. Sustained HBV DNA suppression and ALT normalization were observed in all patients treated with ETV, resulting in significantly higher virologic and biochemical responses compared with LAM. Importantly, no ETV-treated patient developed antiviral resistance throughout the study, confirming that ETV is a potent treatment option with a high barrier to resistance in Korean HBeAg-negative patients with CHB.
ACKNOWLEDGMENTS
This study was sponsored by Bristol-Myers Squibb, Korea. The authors and the sponsor would like to thank all study patients and research staff for their participation in the study. Editorial assistance with the preparation of this manuscript was provided by MediTech Media Asia Pacific and was funded by Bristol-Myers Squibb.
FOOTNOTES
AuthorsŌĆÖ contribution
Kwan Sik Lee, Young-Oh Kweon, Soon-Ho Um, Byung-Ho Kim, Young Suk Lim, Seung Woon Paik, Jeong Heo, Heon-Ju Lee, Dong Joon Kim, Tae Hun Kim, Young-Sok Lee, Kwan Soo Byun, Daeghon Kim, Myung Seok Lee and Dong Jin Suh recruited patients and collected the data. All authors interpreted the data and were involved in development, review, and approval of the manuscript.
Figure┬Ā1.
Flow diagram depicting the enrolment, allocation and progress of patients through the phases of the trial. HBV, hepatitis B virus; ETV, entecavir; LAM, lamivudine; ITT, intent-to-treat.
Figure┬Ā2.
Proportion of patients with virologic response (HBV DNA <300 copies/mL) at weeks 24, 96 and 240. P-values calculated using PearsonŌĆÖs chi-square test (Weeks 24 and 96) or FisherŌĆÖs exact test (week 240). HBV, hepatitis B virus; ETV, entecavir; LAM, lamivudine.
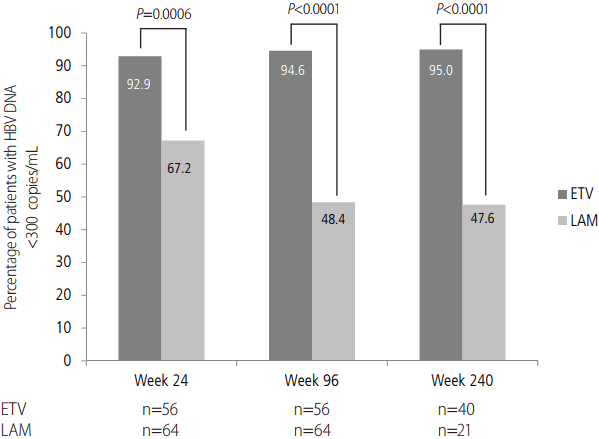
Figure┬Ā3.
Mean log reduction in HBV DNA levels from week 1 to week 240 in ETV- and LAM-treated patients. HBV, hepatitis B virus; ETV, entecavir; LAM, lamivudine.
Table┬Ā1.
Baseline demographics and disease characteristics
| Parameters | ETV (n=56) | LAM (n=64) | P-value | |
|---|---|---|---|---|
| Age, years | Mean┬▒SD | 45.7┬▒11.9 | 49.0┬▒7.8 | 0.09* |
| Median | 48.4 | 50.1 | ||
| Gender, n (%) | Male | 47 (84.0) | 48 (75.0) | 0.23ŌĆĀ |
| Female | 9 (16.0) | 16 (25.0) | ||
| HBV DNA, log10 copies/mL | Mean┬▒SD | 6.1┬▒0.8 | 5.8┬▒0.9 | 0.08* |
| Median | 6.0 | 5.9 | ||
| ALT, IU/mL | Mean┬▒SD | 110.5┬▒82.0 | 93.9┬▒58.5 | 0.38ŌĆĪ |
| Median | 78.0 | 80.0 | ||
| Prior interferon status, n (%) | Yes | 2 (3.6) | 0 (0.0) | 0.22┬¦ |
| No | 54 (96.4) | 64 (100.0) |
Table┬Ā2.
Virologic breakthrough profile
| ETV (n=56) | LAM (n=64) | P-value | |
|---|---|---|---|
| 24 week | |||
| ŌĆān | 54 | 60 | 0.2455ŌĆĀ |
| ŌĆāNegative, n (%) | 54 (100.00) | 57 (95.00) | |
| ŌĆāPositive, n (%) | 0 (0.00) | 3 (5.00) | |
| 48 week | |||
| ŌĆān | 55 | 61 | 0.0042* |
| ŌĆāNegative, n (%) | 54 (98.18) | 50 (81.97) | |
| ŌĆāPositive, n (%) | 1 (1.82) | 11 (18.03) | |
| 96 week | |||
| ŌĆān | 55 | 61 | <0.0001* |
| ŌĆāNegative, n (%) | 54 (98.18) | 35 (57.38) | |
| ŌĆāPositive, n (%) | 1 (1.82) | 26 (42.62) |
Table┬Ā3.
Summary of safety outcomes
| ETV (n=56) | LAM (n=64) | |
|---|---|---|
| Summary of AEs through to Week 240 | ||
| ŌĆāNon-SAEs, n (%) | 48 (85.7) | 49 (76.6) |
| ŌĆāSAEs, n (%) | 7 (12.5) | 17 (26.6) |
| ŌĆāŌĆāInjury, poisoning and procedural complications | 4 (7.1) | 4 (6.3) |
| ŌĆāŌĆāNeoplasms (benign, malignant and unspecified) | 2 (3.6) | 6 (9.4) |
| ŌĆāŌĆāEye disorders | 1 (1.8) | - |
| ŌĆāŌĆāHepatobiliary disorders | 1 (1.8) | 2 (3.1) |
| ŌĆāŌĆāInvestigations | 1 (1.8) | 1 (1.6) |
| ŌĆāŌĆāNervous system disorders | 1 (1.8) | - |
| ŌĆāŌĆāMusculoskeletal and connective tissue disorders | - | 2 (3.1) |
| ŌĆāŌĆāGastrointestinal disorders | - | 1 (1.6) |
| ŌĆāŌĆāInfections and infestations | - | 1 (1.6) |
| ŌĆāŌĆāRespiratory, thoracic and mediastinal disorders | - | 1 (1.6) |
| ŌĆāŌĆāVascular disorders | - | 1 (1.6) |
| ŌĆāSAE related to the study conditions | 0 (0) | 0 (0) |
| ŌĆāDiscontinuations due to AEs | 0 (0) | 1 (1.6) |
| ŌĆāDeath | 1 (1.8)* | 0 (0) |
| Grade 3/4 laboratory abnormalities at Week 96 | ||
| ŌĆāALT | 0 (0) | 6 (9.7) |
| ŌĆāAST | 0 (0) | 3 (4.8) |
| ŌĆāCreatinine | 2 (3.6) | 0 (0) |
| ŌĆāBilirubin | 1 (1.8) | 3 (4.8) |
| ŌĆāGlucose (fasting) | 3 (9.4) | 2 (5.6) |
| ŌĆāLipase | 2 (3.6) | 4 (6.5) |
| ŌĆāPlatelets | 1 (1.8) | 1 (1.6) |
| ŌĆāProthrombin time | 1 (1.8) | 0 (0) |
| ŌĆāNeutrophils | 0 (0) | 1 (1.6) |
Abbreviations
AEs
adverse events
ALT
alanine aminotransferase
APASL
Asian Pacific Association for the Study of the Liver
AST
aspartate aminotransferase
CHB
chronic hepatitis B
CI
confidence interval
ETV
entecavir
HBeAg
hepatitis B e antigen
HBsAg
hepatitis B surface antigen
HBV
hepatitis B virus
LAM
lamivudine
NUC
nucleos(t)ide
PCR
polymerase chain reaction
PEG-IFN
peginterferon
SAEs
serious adverse events
TDF
tenofovir
ULN
upper limit of normal
VR
virologic response
REFERENCES
1. Kim H, Shin AR, Chung HH, Kim MK, Lee JS, Shim JJ, et al. Recent trends in hepatitis B virus infection in the general Korean population. Korean J Intern Med 2013;28:413-419.



2. Chae HB, Kim JH, Kim JK, Yim HJ. Current status of liver diseases in Korea: hepatitis B. Korean J Hepatol 2009;15 suppl 6:S13-S24.


3. Hadziyannis SJ, Vassilopoulos D. Hepatitis B e antigenŌĆÉnegative chronic hepatitis B. Hepatology 2001;34:617-624.


4. Vigan├▓ M, Invernizzi F, Lampertico P. Optimal therapy of chronic hepatitis B: how do I treat my HBeAg-negative patients? Liver Int 2015;35 Suppl 1:107-113.


5. Saikia N, Talukdar R, Mazumder S, Khanna S, Tandon R. Management of patients with HBeAg-negative chronic hepatitis B. Postgrad Med J 2007;83:32-39.



6. Brunetto MR, Oliveri F, Coco B, Leandro G, Colombatto P, Gorin JM, et al. Outcome of anti-HBe positive chronic hepatitis B in alphainterferon treated and untreated patients: a long term cohort study. J Hepatol 2002;36:263-270.


7. Selvaraj V, Wilcox RD. Challenges face clinicians treating HbeAg negative chronic hepatitis B in HIV. HIV Clin 2011;23:1-5.
8. European Association for the Study of the Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol 2012;57:167-185.


9. Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016;10:1-98.



10. Kao JH, Wu NH, Chen PJ, Lai MY, Chen DS. Hepatitis B genotypes and the response to interferon therapy. J Hepatol 2000;33:998-1002.


11. Kwon JH, Kim YS, Kim SG, Jang JW, Kim TH, Jung YK, et al. The efficacy and safety of peginterferon-╬▒-2a in Korean patients with chronic Hepatitis B: A multicenter study conducted in a real clinical setting. Gut Liver 2013;7:197-205.



12. Dienstag JL. Peginterferon therapy for HBeAg-negative chronic hepatitis B: less than meets the eye. Am J Gastroenterol 2010;105:1770-1772.



13. Bae SH, Yoon SK, Jang JW, Kim CW, Nam SW, Choi JY, et al. Hepatitis B virus genotype C prevails among chronic carriers of the virus in Korea. J Korean Med Sci 2005;20:816-820.



14. Kim H, Jee YM, Song BC, Shin JW, Yang SH, Mun HS, et al. Molecular epidemiology of hepatitis B virus (HBV) genotypes and serotypes in patients with chronic HBV infection in Korea. Intervirology 2007;50:52-57.


15. Papatheodoridis GV. Treatment of HBeAg-negative chronic hepatitis B patients with nucleos(t)ide analogues. Liver Int 2011;31 Suppl 1:95-103.


16. Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016;63:261-283.



17. World Health Organization (WHO). WHO Model List of Essential Medicines for Children. (April 2015). WHO website, <http://www.who.int/medicines/publications/essentialmedicines/EMLc2015_8-May-15.pdf>. Accessed 2015.05.08.
18. Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med 2006;354:1001-1010.


19. Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med 2006;354:1011-1020.


20. Lim YS, Han S, Heo NY, Shim JH, Lee HC, Suh DJ. Mortality, liver transplantation, and hepatocellular carcinoma among patients with chronic hepatitis B treated with entecavir vs lamivudine. Gastroenterology 2014;147:152-161.


21. Luo J, Li X, Wu Y, Lin G, Pang Y, Zhang X, et al. Efficacy of entecavir treatment for up to 5 years in nucleos(t)ide-naïve chronic hepatitis B patients in real life. Int J Med Sci 2013;10:427-433.



22. Shouval D, Lai CL, Chang TT, Gadano A, Wu SS, Halota W. Three years of entecavir re-treatment of HBeAg (-) entecavir patients who previously discontinued entecavir therapy: results from study ETV-901 [Abstract]. Hepatology 2008;48(Suppl 1):722A-723A.
23. Myung HJ, Jeong SH, Kim JW, Kim HS, Jang JH, Lee DH, et al. Efficacy and predictors of the virologic response to entecavir therapy in nucleoside-naive patients with chronic hepatitis B. Korean J Hepatol 2010;16:57-65.


24. Huang YH, Wu JC, Chang TT, Sheen IJ, Lee PC, Huo TI, et al. Analysis of clinical, biochemical and viral factors associated with early relapse after lamivudine treatment for hepatitis B e antigen-negative chronic hepatitis B patients in Taiwan. J Viral Hepat 2003;10:277-284.


25. Bang SJ, Kim BG, Shin JW, Ju HU, Park BR, Kim MH, et al. Clinical course of patients with insufficient viral suppression during entecavir therapy in genotype C chronic hepatitis B. Dig Liver Dis 2013;45:600-605.


26. Seto WK, Hui AJ, Wong VW, Wong GL, Liu KS, Lai CL, et al. Treatment cessation of entecavir in Asian patients with hepatitis B e antigen negative chronic hepatitis B: a multicenter prospective study. Gut 2015;64:667-672.


27. Shouval D, Lai CL, Chang TT, Cheinquer H, Martin P, Carosi G, et al. Relapse of hepatitis B in HBeAg-negative chronic hepatitis B patients who discontinued successful entecavir treatment: the case of continuous antiviral therapy. J Hepatol 2009;50:289-295.


28. Tenney DJ, Pokornowski KA, Rose RE, Baldick CJ, Eggers BJ, Fang J, et al. Entecavir maintains a high genetic barrier to HBV resistance through 6 years in naïve patients. J Hepatol 2009;50:S10.

29. Lai CL, Dienstag J, Schiff E, Leung NW, Atkins M, Hunt C, et al. Prevalence and clinical correlates of YMDD variants during lamivudine therapy for patients with chronic hepatitis B. Clin Infect Dis 2003;36:687-696.



- TOOLS
-
METRICS

- ORCID iDs
-
Kwan Sik Lee

https://orcid.org/0000-0002-3672-1198 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



