INTRODUCTION
After anticonvulsant medication, a specific hyperreactivity can develop into anticonvulsant hypersensitivity syndrome that shows clinical characteristics such as fever, rash, and internal organ invasion. Among the internal organ invasion, liver invasion is the most common symptom.1 The anticonvulsant hypersensitivity syndrome mostly occurs after aromatic anticonvulsant medication such as phenytoin, phenobarbital, carbamazepine, and lamotrigine.2 In particular, lamotrigine that is generally used for disorder such as absence epilepsy, shows common light symptoms such as fever and rash, and rarely reported severe cases that rapidly progressed with interstitial pneumonia or hepatic failure.345678 The case report herein presents an example of a patient who was administered with valproic acid for convulsion control and developed hepatic failure after additional dose of lamotrigine, which led up to death.
CASES
A 33-year-old male patient was hospitalized in Bundang Jeasang General Hospital for the symptoms of fever and skin rash that lasted for the past three days. On the admission day, the rashes of round lesions about an inch in diameter were observed over the whole body. His medical history included epilepsy associated with traumatic fracture compound comminuted depressed since 21 years. The patient had taken 1,200 mg/day of valproic acid, but he still experienced the seizure. 100 mg/day of lamotrigine was also taken for 15 days before visiting our hospital. He was administered 2.5 mg/day of aripiprazole for post-traumatic organic brain syndrome. Physical examination showed that he was alert and well-oriented; he had a diffuse erythema multiforme about an inch without itching, mucosal edema of the mouth and conjunctivitis of the eyes; his temperature was 40.6Ōäā. Laboratory data showed white cells, 12.0├Ś103/mm3 (74.8% neutrophils, 13.9% lymphocytes, 3.5% eosinophils); hemoglobin, 15.3 g/dL; platelet, 35├Ś103/mm3; aspartate aminotransferase, 247 IU/mL; alanine aminotransferase, 58 IU/mL; total bilirubin, 0.4 mg/dL; prothrombin time, 58.8%; international normalized ratio, 1.30; protein, 6.4 g/dL; albumin, 3.7 g/dL; alkaline phosphatase, 379 IU/L, ╬│-glutamyl transpeptidase, 62 IU/L; blood urea nitrogen, 16.6mg/dL; creatinine, 1.49 mg/dL. The results of serological tests including for HBsAg, anti-HCV, HCV-RNA, anti HAV-IgM, Epstein-Barr virus VCA-IgM, heterophil antibody and HIV were negative. Abdominal ultrasound showed a mildly fatty liver.
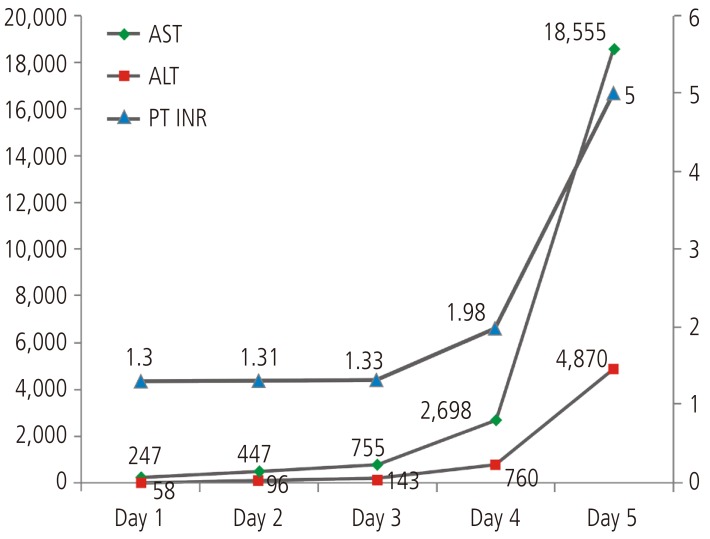
Lamotrigine was immediately discontinued, and levetiracetam was tried in monotherapy for epilepsy. Dexamethasone and antihistamine were applied. The skin lesion appeared improved, however the liver function did not improve. On the 3rd day, he had suffered from consciousness degradation. On the 5th day of admission, he was comatose and his laboratory results were: total bilirubin 1.83 mg/dL, alanine aminotransferase 15,975 IU/L, aspartate aminotransferase 4,730 IU/L, alkaline phosphatase 3,333 IU/L, ╬│-glutamyl transpeptidase 147 IU/L, prothrombin time 0%, serum albumin 3.3 g/dL, and creatinine 1.80 mg/dL (Fig. 1). Hepatic encephalopathy and coagulopathy had progressed and the patient died 5days after admission due to hepatic failure.
DISCUSSION
Lamotrigine is a commonly used anticonvulsant drug that is superior in absorption with relatively less interaction with other medications. It is aromatic phenyltriazine with two benzene rings, displaying linear pharmacokinetic characteristics. The anticonvulsant effects are achieved as it stabilizes synaptic membrane through glutamate release and calcium channel control by blocking voltage dependent sodium channel and suppressing secretion of excitable neurotransmitter that serves in N-methyl-D-aspartate receptor.910
Lamotrigine can cause some adverse effects such as headache, nausea, dizziness, skin rash, acute poisoning symptoms in nervous system such as epileptic seizure, delirium, hypertonia, arrhythmia such as ventricular tachycardia, and electrocardiographic QRS widening. Other severe side effects include Stevens-Johnson syndrome, toxic epidermal necrolysis, pneumonitis, interstitial nephritis, encephalitis, and pericarditis.1
For liver invasion caused by lamotrigine, wide spectrums of symptoms were reported, ranging from mild liver enzyme elevation to fulminant hepatic failure.34567 In case of liver failure, liver enzyme elevation mostly occurs in patients who are administered lamotrigine, particularly when the dosages were increased one or two weeks before visiting a hospital. When the medication was stopped, many of them showed improvement in blood tests and clinical characteristics. For rapid progression caused by hepatic failure, there is one case reported by Ouellet et al.8 in 2009.8 When the symptoms of anticonvulsant hypersensitivity syndromes occurred, the dose of lamotrigine was from 5 mg at the lowest to 400 mg at the highest in many case reports; the occurrence of the anticonvulsant hypersensitivity syndromes does not seem to bear proportional relationship to the dosage. According to previous reports, hypersensitivity reaction occurred during administering lamotrigine alone, and in a few cases of severe progression, acute hepatitis was correlated with combined treatment with different medication such as lamotrigine and valproic acid.4567 Lamotrigine is metabolized by the glucuronic acid metabolism in the liver, and the metabolized 43-87% of glucuronide conjugate dosage, is excreted through urine. Although the half-life is known as 22.7-37.4 hours, it is extended to 48.3-59 hours in combined treatment with valproic acid, and reduced to 13.5-15 hours in combined treatment with phenytoin, phenobarbital, and carbamazepine. One possible explanation is that valproic acid reduces clearance of lamotrigine through this metabolism, which causes hepatotoxicity.11 4-ene-VPA, metabolite of valproic acid, suppresses B-oxidization, and the metabolite is formed by cytochrome P450 enzyme, which increases hepatotoxicity when it is used with anticonvulsant inducing liver enzyme. Typically, liver enzyme increases, but severe hepatotoxicity is rare.121314
Development of hypersensitivity reaction to aromatic anticonvulsant such as lamotrigine-induced reaction is a very rare with 0.001-0.0001% frequency. It usually takes 2-6 weeks after administering anticonvulsant to develop the clinical characteristics such as fever, rash, and internal organ invasion such as liver and lung. The cytotoxicity is caused by the metabolites of aromatic anticonvulsant such as arene oxide. Although epoxide hydrolase degrades arene oxides, a deficit of the enzyme can cause anticonvulsant hypersensitivity syndromes.1516
In most case reports for the combined treatment with valproic acid the recovery was observed within 5 days to 6 weeks after stopping lamotrigine, the liver functions were also recovered. A case report by Overstreet et al3 showed a rare case where a patient was treated by combined medication with acetaminophen, olanzapine, and risperidone, which resulted in death by hepatic failure.
In the herein, although skin symptoms improved by conducting principled management and treatment for patients such as intravenous corticosteroid for the Stevens-Johnson syndrome, irreversible hepatic failure was developed, which led to death. As lamotrigine is known as a secured drug, it needs to be administered to the patients who are not controlled with general anticonvulsants or initial treatment. However, it is important to note that lamotrigine can develop fatal hepatic failure in combination with a drug conducting metabolism through cytochrome P450 conduit. Furthermore, regular liver function tests are needed as shown by the case of hepatic failure. The case report herein aims to draw attention to rapid clinical measures such as modified or changed medication when it comes to numerical value elevation on liver enzyme in the test results.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




