| Clin Mol Hepatol > Volume 27(1); 2021 > Article |
|
ABSTRACT
Background/Aims
Methods
Results
ACKNOWLEDGMENTS
FOOTNOTES
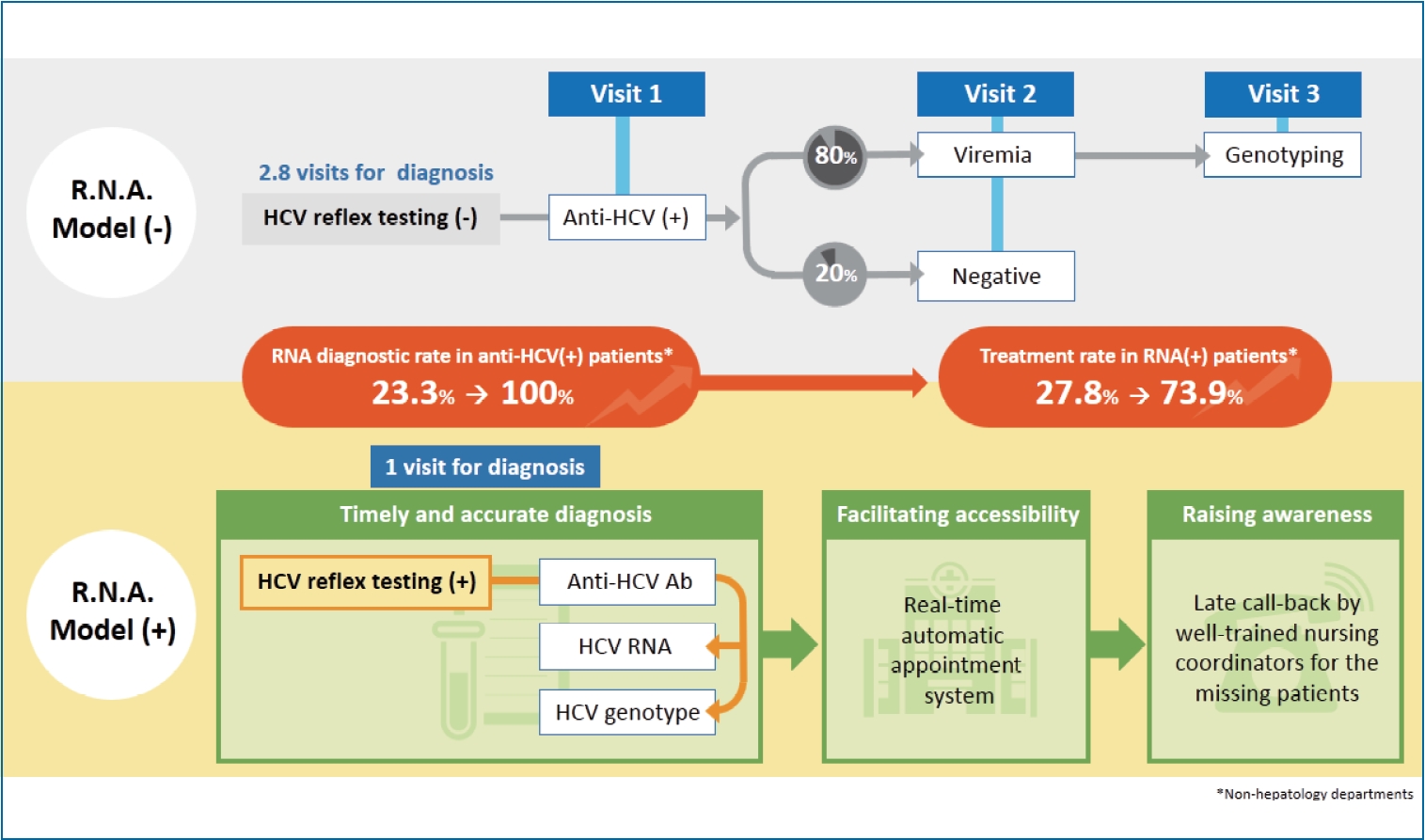
Figure 1.
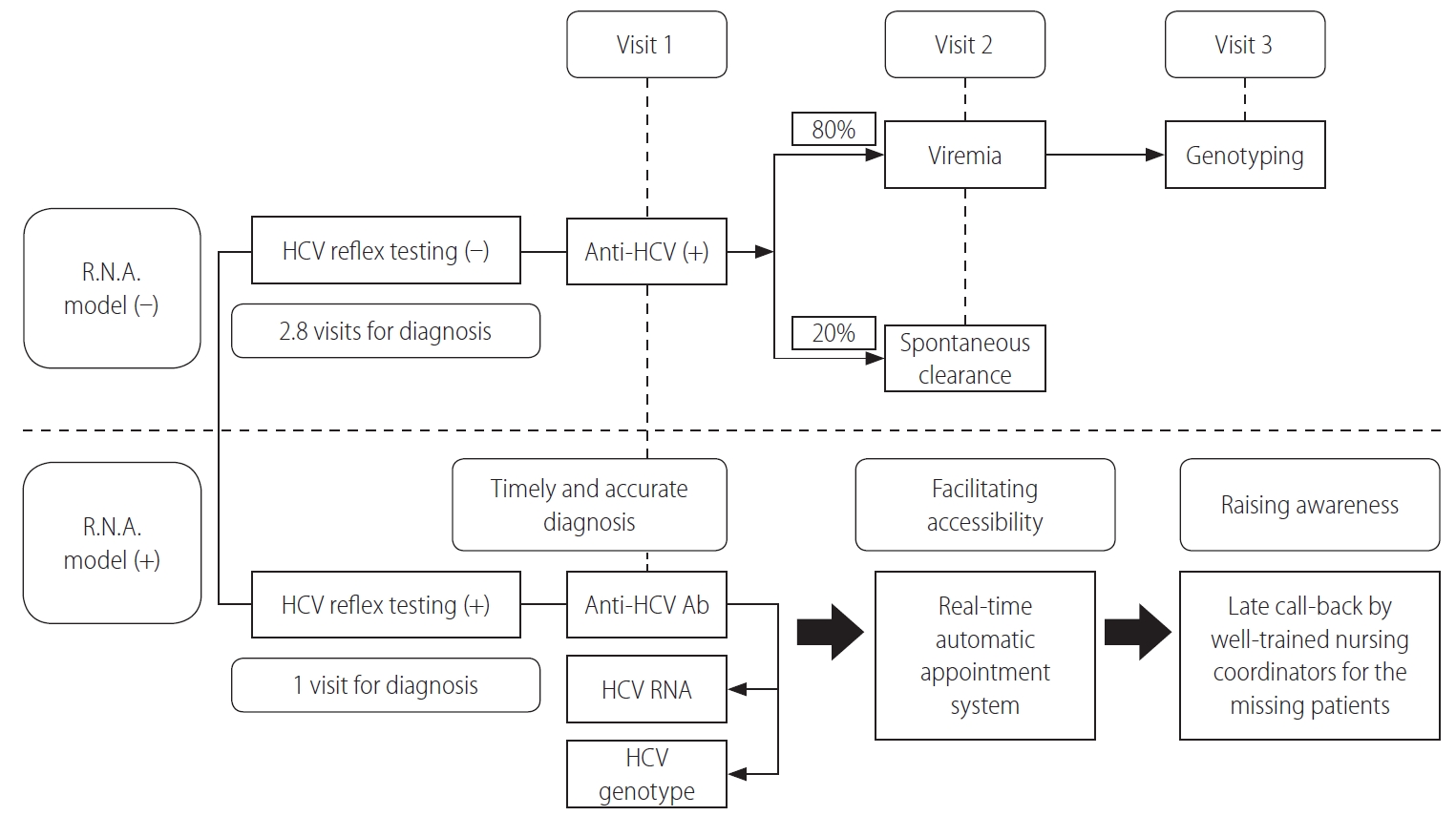
Table 1.
| R.N.A model (-) (n=1,396) | R.N.A. model (+) (n=125) | P-value | |
|---|---|---|---|
| Male gender | 675 (48.4) | 74 (59.2) | 0.02 |
| Age (years) | 58.9±13.3 | 58.6±14.8 | 0.27 |
| Patient source | <0.001 | ||
| Hepatology department | 1,139 (81.6) | 68 (54.4) | |
| Non-hepatology department* | 257 (18.4) | 57 (45.6) | |
| Diabetes | 233 (16.7) | 22 (17.6) | 0.79 |
| Hypertension | 469 (33.6) | 50 (40.0) | 0.15 |
| Cardiovascular disease | 44 (3.2) | 6 (4.8) | 0.30 |
| Cerebrovascular disease | 80 (5.7) | 8 (6.4) | 0.76 |
| White blood cell (/mm3) | 5,969±1,931 | 6,199±2,228 | 0.41 |
| Hemoglobin (g/dL) | 13.8±1.8 | 13.5±1.9 | 0.32 |
| Platelet count (×1,000/mm3) | 203±70 | 198±77 | 0.60 |
| AST (IU/L) | 57.0±44.9 | 61.4±41.8 | 0.41 |
| ALT (IU/L) | 66.6±70.6 | 74.4±63.9 | 0.34 |
| Creatinine (mg/dL) | 1.27±1.79 | 1.14±1.22 | 0.41 |
| HCV RNA† (log IU/mL) | 5.69±1.13 | 5.48±1.23 | 0.18 |
| HCV genotype 1†, n/N (%) | 493/948 (52.0) | 54/100 (54.0) | 0.70 |
| DAA regimen‡ | <0.001 | ||
| EBR/GZR | 156 (17.2) | 1 (1.2) | |
| SOF/LDV | 311 (34.2) | 0 (0.0) | |
| GLE/PIB | 304 (33.5) | 32 (38.6) | |
| SOF/VEL | 137 (15.1) | 50 (60.2) |
Values are presented as mean±standard deviation or number (%) unless otherwise indicated.
HCV, hepatitis C virus; R.N.A. model, HCV Reflex testing; Call-back by Nursing coordinators; Automatic appointment system; AST, aspartate aminotransferase; ALT, alanine aminotransferase; DAA, direct-acting antiviral; EBR, elbasvir; GZR, grazoprevir; SOF, sofosbuvir; LDV, ledipasvir; GLE, glecaprevir; PIB, pibrentasvir; VEL, velpatasvir.
* Including Division of Infectious Diseases (n=54), Department of Otolaryngology (n=53), Department of Psychiatry (n=49), Department of Surgery (n=43), Division of Nephrology (n=31), Division of Endodontics and Operative Dentistry (n=18), Division of Pulmonary Medicine (n=15), Department of Family Medicine (n=13), and others (n=38).
Table 2.
| R.N.A. model (-) | R.N.A. model (+) | P-value | |
|---|---|---|---|
| RNA testing in anti-HCV+ patients | 84.8% (1,184/1,396) | 100.0% (125/125) | <0.001 |
| Hepatology department | 98.7% (1,124/1,139)* | 100.0% (68/68) | 0.999 |
| Non-hepatology department | 23.3% (60/257)* | 100.0% (57/57) | <0.001 |
| HCV genotyping in HCV RNA+ patients | 98.9% (938/948) | 100.0% (100/100) | 0.61 |
| Hepatology department | 99.1% (922/930) | 100.0% (54/54) | 0.999 |
| Non-hepatology department | 88.9% (16/18) | 100.0% (46/46) | 0.08 |
| Treatment rate in HCV RNA+ patients | 95.8% (908/948) | 83% (83/100) | <0.001 |
| Hepatology department | 97.1% (903/930)* | 96.1% (49/51)†,‡ | 0.66 |
| Non-hepatology department | 27.8% (5/18)* | 73.9% (34/46)† | 0.001 |
| HCV treatment uptake§ | 81.2% | 83.0% | 0.85 |
| Hepatology department | 95.8% | 96.1% | 0.76 |
| Non-hepatology department | 6.4% | 73.9% | <0.001 |
HCV, hepatitis C virus; R.N.A. model, HCV Reflex testing; Call-back by Nursing coordinators; Automatic appointment system.
REFERENCES
- TOOLS



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





