INTRODUCTION
Intrahepatic and extrahepatic cholangiocarcinomas develops through a multistep carcinogenesis sequence and they are preceded by precursor lesions. Precursors of malignant neoplasm are identified in various organs including uterine cervix, gastrointestinal tract and breast. Identification of these precursor lesions are important in that they need minimal treatment or they are good indicator of disease progression or recurrence.
In the intrahepatic and extrahepatic biliary tract, precursor lesions of malignancy are defined as follows; biliary intraepithelial neoplasia (BilIN), intraductal papillary neoplasm, mucinous cystic neoplasm, and adenoma.1 BilIN is characterized by atypical epithelial cells with multi-layering of nuclei and micropapillary projections into the lumen of bile duct.2,3 In contrast to intraductal papillary neoplasm which is characterized by papillary proliferation of epithelial cells with fibrovascular stroma and mucin production, BilIN tend to be a microscopic lesion. Mucinous cystic neoplasm is usually cystic lesions and has distinguishing clinicopathologic findings such as subepithelial stroma resembling ovarian stroma and predominant occurrence in adult female. Adenomas are commonly encountered in extrahepatic bile ducts and divided into tubular, papillary and tubulopapillary on the basis of growth pattern.1
CASE SUMMARY
A 70-year-old previously healthy female presented with general weakness. She is a carrier of hepatitis B virus, but has no recent history of hepatitis. The results of a physical examination were unremarkable. Initial serum aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase levels were 52 IU/L, 24 IU/L and 47 IU/L, respectively. Serologic marker for hepatitis B surface antigen was positive. Serum alpha-fetoprotein, carcinoembryonic antigen and carbohydrate antigen 19-9 levels were 2.3 ng/mL, 1.2 ng/mL and 7.1 U/mL, respectively. Abdominal ultrasonography and computed tomography revealed dilatation of left intrahepatic bile ducts with focal narrowing of left hepatic duct. Because the possibility of cholangiocarcinoma could not be excluded, the patient submitted to a left lobectomy of liver.
PATHOLOGIC FINDINGS
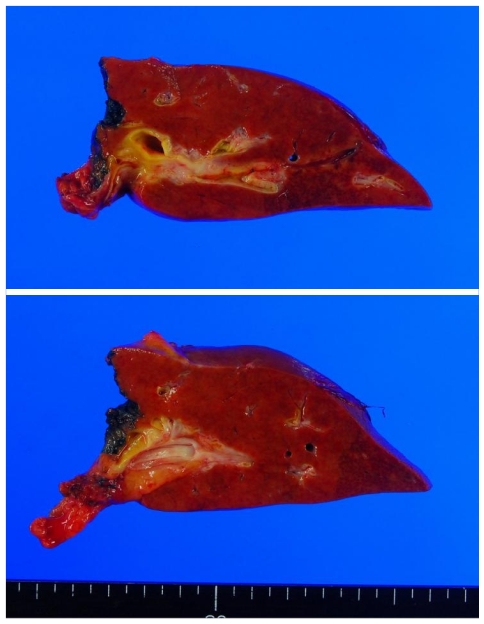
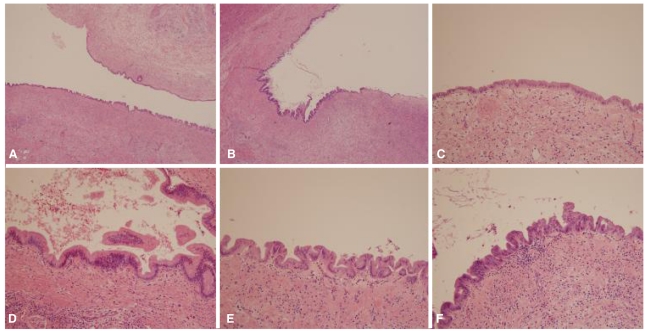
On gross examination, liver surface was unremarkable. When bisected along hepatic duct, intrahepatic duct showed cystic dilatation (Fig. 1). Extrahepatic bile duct demonstrated focal narrowing of lumen, but no mass lesion was observed. Microscopically, there was wall thickening with fibrous change at extrahepatic bile duct portion. Cystically dilated intrahepatic bile duct was covered by biliary epithelia showing variable degree of atypia (Fig. 2). Epithelial cells lining these lesions were mostly flat, but in some foci, they showed micropapillary or tufted appearance. Pseudostratification of nuclei was commonly finding. Variations in nuclear size, nuclear membrane irregularity and abnormally large nuclei are also noted. Invasion of basal lamina was not observed. This patient was finally diagnosed as biliary intraepithelial neoplasia.
DISCUSSION
BilIN is not infrequently encountered in routine pathologic practice. In surgically removed liver samples of chronic biliary disease and chronic liver disease such as hepatolithiasis, primary sclerosing cholangitis, choledochal cyst, chronic hepatitis C, and alcoholic cirrhosis, BilINs can be found without difficulty and in which condition, the incidence of cholangiocarcinoma is high.3-5 BilINs are also frequently found in adjacent to invasive cholangiocarcinomas and the incidence of BilIN-3 lesion parallels that of invasive cholangiocarcinoma suggesting that BilIN could be precursor lesion of invasive cholangiocarcinoma.1 There is a report about progression of BilIN to invasive cholangiocarcinoma in which immunohistochemical staining of p21, p53 and cyclin-D1 antibodies revealed up-regulation of these proteins with histological progression from BilIN to invasive cholangiocarcinoma.6 A molecular study also revealed stepwise increase of promoter CpG island methylation and decrease of repetitive element methylation in BilIN-cholangiocarcinoma sequence.7
BilIN is usually not recognized on macroscopic examination. On macroscopic examination, mucosa shows only discolored, granular or plaque-like appearance. Microscopically, BilIN shows enlargement of cells, pseudostratification and micropapillae formation.1-3 According to the degree of cytological and architectural atypia, BilINs are divided into BilIN-1, BilIN-2 and BilIN-3. BilIN-1 correspond to low grade lesion (ICD-O code, 8148/0), BilIN-2 to intermediate lesion (ICD-O code, 8148/0) and BilIN-3 to high grade lesion (ICD-O code, 8148/2).1 Some of hyperplastic or regenerative change could show cytological and structural features resembling BilINs, but these lesions lack sufficient atypia to be regarded as neoplastic.
Precursor lesions of intra- and extrahepatic biliary tract are not easily accessible due to the anatomic location, so it is difficult to get biopsy samples through endoscopic or needle biopsy. Furthermore, BilIN is mostly flat or micropapillary lesion that neither form mass lesion nor cause bile duct obstruction. Early detection or regular follow up of BilIN is very difficult. This is why the natural course or prognosis of BilIN is not well understood. Till now, the knowledge and clinical utilization of BilIN is limited. But it can provide the information about carcinogenesis of cholangiocarcinoma and this classification of precursor lesion of malignancy could be applicable to clinical or pathologic studies of biliary epithelium and to evaluation of patients with hepatolithiasis, choledocal cyst and primary sclerosing cholangitis patients. So, it is hoped that understanding of BilIN could be able to help us better understand of biliary tract neoplasia.
This case shows variable degree of BilINs at cystically dilated intrahepatic bile duct proximal to benign stricture of extrahepatic bile duct. These findings suggest that chronic stimuli at bile duct epithelium could promote dysplastic change and these stimuli may be the cause of development of cholangiocarcinoma.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




