Current status of and strategies for hepatitis C control in South Korea
Article information
Abstract
Chronic hepatitis C (CHC) is caused by hepatitis C virus (HCV) infection. HCV infection causes acute hepatitis, and the majority of those infected progress to chronic hepatitis, and some of them develop cirrhosis and hepatocellular carcinoma. Transmission of HCV is parenteral, and the major transmission routes include drug abuse, insecure injections or medical procedures, contaminated syringes or needles, sexual contact with an HCV-infected person, vertical infection of newborns by infected mothers, the transfusion of blood or blood products contaminated with viruses, and organ transplants. As no vaccine against HCV is available, HCV management involves blocking routes of transmission transmission, screening for HCV infection, and protecting liver disease progression by treatment. Highly potent oral direct antiviral agents are now available. Therefore, early detection through nation-wide screening program and appropriate treatment should be implemented to improve the quality of life of patients with HCV. Furthermore, for the effective HCV control in South Korea, The organization of an ‘integrated national viral hepatitis control system’ is desirable.
INTRODUCTION
Hepatitis C virus (HCV) infection causes chronic hepatitis in most patients, and some of them eventually develop cirrhosis or hepatocellular carcinoma [1]. It is estimated that approximately 3% of the world’s population are infected with HCV and chronic hepatitis C (CHC) accounts for about 15–20% of all chronic liver disease in South Korea. Transmission of HCV is parenteral, and the major transmission routes include drug abuse, insecure injections or medical procedures, contaminated syringes or needles, sexual contact with an HCV-infected person, vertical infection of newborns by infected mothers, the transfusion of blood or blood products contaminated with viruses, and organ transplants [2]. Between 2015 and 2016, mass outbreaks of HCV occurred at several hospitals in Seoul and other provincial clinics, primarily due to reuse of disposable syringes.
As an effective HCV vaccine has not yet been discovered, public health considerations underscore the need to develop both an understanding of the national epidemiology of HCV infection and a preventive strategy to block routes of this condition.
EPIDEMIOLOGY
Routes of transmission
Transmission of HCV is parenteral, and a screening test for blood donors was introduced in 1991. Therefore, transmission via transfusion was not a major route of infection after 1992 [3-5]. The use of illicit drugs is a well-known risk factor for HCV infection; indeed, the proportion of HCV infection among drug users is approximately 80% in South Korea [5,6]. However, a nationwide prospective cohort study that included 1,173 subjects revealed that the proportion of drug abuse was 5% [7]. Thus, identification of other routes of transmission is necessary. According to a study of 207 patients with chronic HCV infection, the risk factors for HCV infection include older age, needle-stick injury, dental procedures, multiple sex partners (≥4), blood transfusion before 1991, and surgery [8]. A comparative study of 1,173 HCV patients and 534 controls in five university hospitals between 2007 and 2011 in South Korea reported several independent risk factors for infection, including use of illicit drugs, needle-stick injury, transfusion before 1995, tattoo, and age [7].
Prevalence in adult health-check examinees
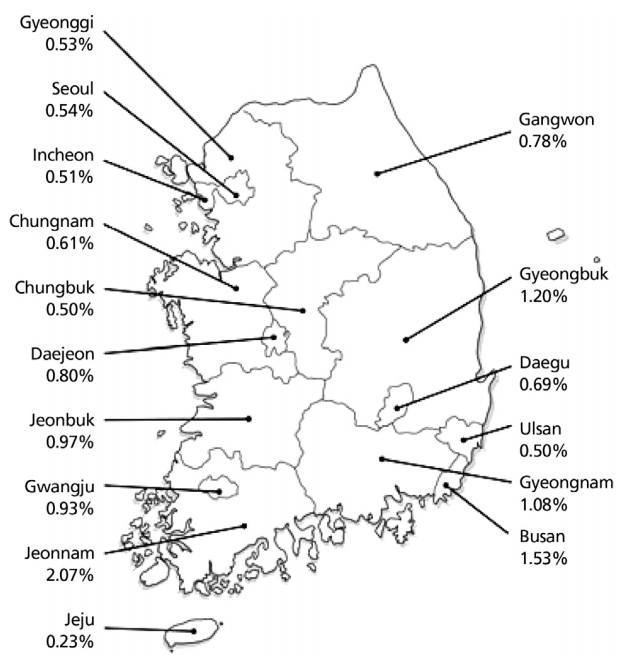
The estimated age-standardized prevalence of anti-HCV in adult health-check examinees > 40 years of age is 1.29% (95% confidence interval, 1.12–1.48) according to a collective study of health-check examinees from Seoul, Ulsan, Jeollanam-do, and Daegu between 1995 and 2000 [6,9-12]. In 2009, the prevalence of anti-HCV was 0.78% in 291,314 patients older than 20 years who underwent health check-up at 29 health examination centers using the 3rd generation enzyme immunoassay (EIA) after adjusting for age, sex, and area. In that study, the prevalence of anti-HCV was higher in females (0.83%) than in males (0.75%) and increased with age (20–29 years, 0.34%; 30–39 years, 0.41%; 40–49 years, 0.60%; 50–59 years, 0.80%; 60–69 years, 1.53%; and ≥ 70 years, 2.31%) [3]. Additionally, the anti-HCV prevalence varied geographically; in comparison with the prevalence of 0.50–1.20% in most regions, including Seoul and Gyeonggi-do, the prevalence in Pusan and Jeollanam-do were 1.53% and 2.07%, respectively. The Jeju Special Self-Governing Province had the lowest prevalence, 0.23% (Fig. 1) [3].
The Korea National Health and Nutrition Examination Survey has included anti-HCV testing since 2012. From 2012 to 2014, the prevalence of hepatitis C antibody positivity was 0.7% among 15,795 subjects aged ≥19 years, which was similar to that in the 2009 National Health Survey (Table 1). The prevalence was low (0.4%) in subjects with a high level of income [13].

The prevalence of hepatitis C antibody positivity from the Korea National Health and Nutrition Examination Survey for the period from 2012 to 2014 from the Korea National Health and Nutrition Examination Survey (unit: %)
According to the infectious disease statistics of the Korea Center for Disease Control [14], the average number of reports of hepatitis C infection in 2015 was 4,205 (standard deviation, 331.1). Up to the end of August 2016, 4,003 cases had been reported; therefore, the number of reports has increased within the past 2 years (Table 2). However, according to the 2014 annual surveillance report of the Korea Center for Disease Control [15], the number of reports per institution has remained relatively stable, at 43.2 in 2011 and 35 in 2014 (Fig. 2).
Prevalence of anti-HCV in high-risk groups
High-risk groups for HCV infection include people who inject drugs, patients under hemodialysis, and those with human immunodeficiency virus (HIV) infection, hemophilia, and leprosy. However, the majority of studies of the prevalence of HCV in these groups were conducted prior to 2000; few studies were performed thereafter [2].
According to a 1997 survey, the domestic anti-HCV prevalence among intravenous drug users was 79.2% [16]. From 2007 to 2010, the rates of hepatitis C antibody positivity and HCV ribonucleic acid (RNA) positivity among the HCV RNA-positive subjects among 318 intravenous drug abusers in South Korea were 48.4% and 98.1%, respectively [17]. The anti-HCV prevalence was 5.9–14.7% in previous studies of >200 patients with chronic kidney disease who underwent hemodialysis between 1997 and 1998 [18,19]. According to the 2014 report of the Korean Society of Nephrology [20], the hepatitis C antibody positivity rate was 2.2% and was correlated with the duration of hemodialysis. The co-infection of HCV and HIV was relatively high, which accounts for 5.0-6.3% of HIV infected patients in South Korea [21-23]. According to a 2002 report, the anti-HCV prevalence in hemophilia patients was 42.3%, and the risk of infection was correlated with age and severity of hemophilia [24]. In their 2012 annual report, the Korea Hemophilia Foundation reported that the rates of anti-HCV antibody positivity and HCV RNA positivity were 20.0% and 5.5%, respectively [25]. The anti-HCV prevalence tested by second-generation EIA was 67.7% in 1997; 82% of these individuals were immunoblot-positive [26]. According to 2009 data, the anti-HCV prevalence and the HCV RNA positivity rate among those anti-HCV positive were 35.1% and 88%, respectively [27].
Strategies for HCV control in South Korea
As seen in the recent ‘HCV infection events’ in the Dana and Hanyang clinics, there is a problem with the management and inspection of the risk of viral hepatitis transmission in front-line medical institutes. Unlike hepatitis B, hepatitis C can be cured by antiviral treatment, and the sustained virologic response rate of this treatment is very high with few side-effects. Therefore, in Korea, if suspected, a hepatitis C test should be performed and treated early. Therefore, it is necessary to evaluate the national management system for hepatitis C, and a system of detection and reporting similar to those implemented by advanced countries should be developed.
At present, 186 medical institutions subject to the government’s selected monitoring are obliged to report cases of HCV to health authorities (Table 3). Therefore, HCV cases in medical institutes other than the 186 designated institutes can be missed. In November 2016, a new law which designated hepatitis C as a group 3 nationally notifiable infectious disease and mandated a total reporting system for HCV infection had been passed. This new law will come into effect on June. 3rd in 2017.
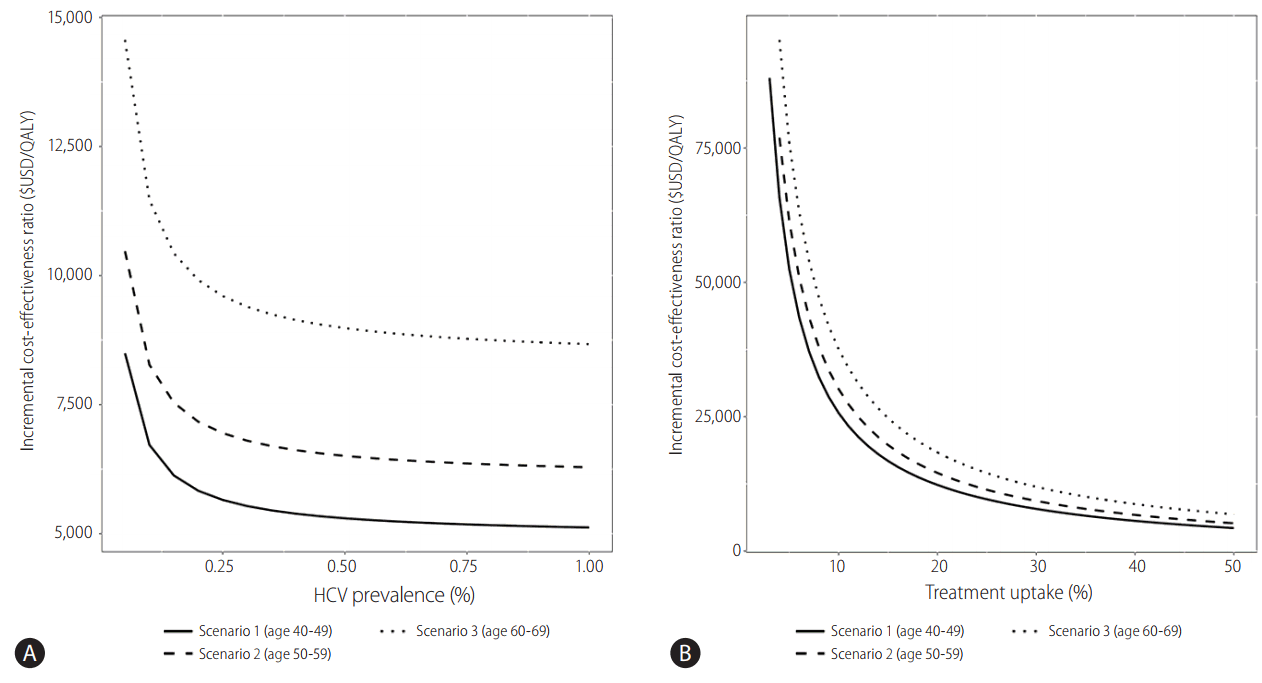
National screening for HCV infection has not yet been implemented in South Korea, because its prevalence is relatively low compared to that of HBV infection. In contrast to HBV infection, HCV infection can be cured by administration of direct acting antivirals. Therefore, scholars believe that a national screening program for HCV should be implemented to facilitate prompt treatment. From the viewpoint of health economics, early treatment of HCV infection prior to progression of liver disease might reduce the overall medical cost. One recent study used Markov modeling to assess the cost-effectiveness of antiviral treatment for patients in the general population diagnosed with hepatitis C [28]. In that study, regardless of risk factors, hepatitis screening tests for HCV in 40-, 50-, and 60-year-olds reduced the incidence of liver cirrhosis and liver cancer by inhibiting the progression of liver disease. One-time HCV screening and treatment in South Koreans aged 40–70 years is likely to be highly cost-effective compared to the current practice not conducting screening [28]. Screening resulted in the identification of 43,635 previously undiagnosed patients across all cohorts. One-time HCV screening and treatment was estimated to be cost-effective across all cohorts, and predicted incremental cost-effectiveness ratios (ICERs) ranged from $5,714 to $8,889 per quality-adjusted life year (QALY) gained. Incremental costs associated with screening, treatment, and disease management ranged from $156.47 to $181.85 million USD; lifetime costs-offsets associated with the avoidance of end-stage liver disease complications ranged from $51.47 to $57.48 million USD [28]. The relationship among HCV prevalence, treatment uptake, and the cost-effectiveness of the screening and treatment program is shown in Fig. 3. Screening and treatment remained cost-effective at a $27,512/QALY threshold across all scenarios in which HCV prevalence was at least 0.04%. In the analysis using baseline HCV prevalence, screening remained cost-effective when treatment uptake was at least 11%, 12%, and 15% in the 40–49-year-old, 50–59-year-old, and 60–69-year-old cohorts, respectively. The relationship between the timing of treatment after diagnosis and its incremental costs and QALY gains is presented in Table 4. Across all scenarios, treating patients sooner after diagnosis was associated with reduced total cost and increased QALY gains compared to base case analyses in which patients were treated over a 5-year time horizon. Decreasing the delay before treatment initiation increased cost-effectiveness estimates across all age groups.
Unlike Western countries, in which the major transmission route is illegal IV drug use, the prevalence of hepatitis C in South Korea is high in the elderly population, especially in coastal areas [8]. Additionally, the frequencies of a history of acupuncture, moxibustion, and tattooing were higher in patients with hepatitis C. Moreover, workers in dental clinics, Oriental clinics, acupuncture centers, and tattoo parlors had low levels of awareness of infection control and are not obliged to report HCV infection. In the past 2 years, the media has reported three outbreaks of infectious diseases originating in medical institutions. Such outbreaks are presumed to be due to syringe reuse. Therefore, members of the general population who do not have high-risk diseases (including HIV, hemophilia, leprosy, and chronic kidney disease) could be exposed to HCV infection in medical institutions in which invasive procedures are performed. Therefore, practical measures for infection control should be implemented in these institutes. Also, appropriate supervision by health authorities of medical institutions in which invasive procedures are performed is warranted. Education programs regarding the medical hazards of CHC and how to prevent transmission are necessary not only for the general population but also for medical personnel, including physicians and paramedics.
Furthermore, establishment of an independent ‘viral hepatitis sector’ in Korea Center for Disease Control & Prevention is recommended to implement a strategy for the management of viral hepatitis. As a control tower has paramount importance in providing health care strategy into practice, organization of an ‘integrated national viral hepatitis control system’ is proposed.
CONCLUSION
As no vaccine against HCV is available, HCV management involves blocking routes of transmission, screening for HCV infection, and preventing liver disease progression by treatment. Highly potent oral direct antiviral agents are now available. Therefore, early detection through national screening and appropriate treatment should be implemented to improve the quality of life of patients with HCV.
Abbreviations
CHC
chronic hepatitis C
EIA
enzyme immunoassay
HCV
hepatitis C virus
HIV
human Immunodeficiency Virus
ICER
incremental cost-effectiveness ratio
QALY
quality-adjusted life year
RNA
ribonucleic acid
Notes
Conflicts of Interest: The authors have no conflicts to disclose.
Funding support
This research was supposed by a fund (4851-308-260-01) by Research of Korea Centers for Disease Control and Prevention.