Regression of esophageal varices and splenomegaly in two patients with hepatitis-C-related liver cirrhosis after interferon and ribavirin combination therapy
Article information
Abstract
Some recent studies have found regression of liver cirrhosis after antiviral therapy in patients with hepatitis C virus (HCV)-related liver cirrhosis, but there have been no reports of complete regression of esophageal varices after interferon/peg-interferon and ribavirin combination therapy. We describe two cases of complete regression of esophageal varices and splenomegaly after interferon-alpha and ribavirin combination therapy in patients with HCV-related liver cirrhosis. Esophageal varices and splenomegaly regressed after 3 and 8 years of sustained virologic responses in cases 1 and 2, respectively. To our knowledge, this is the first study demonstrating that complications of liver cirrhosis, such as esophageal varices and splenomegaly, can regress after antiviral therapy in patients with HCV-related liver cirrhosis.
INTRODUCTION
The benefit of sustained virologic response (SVR) after interferon-based antiviral therapy, defined as HCV RNA negative for 24 weeks after cessation of treatment [1], in patients with chronic hepatitis C (CHC) is well-documented [2,3]. Recent studies also suggest that liver cirrhosis is reversible after antiviral therapy in patients with CHC [4-6]. However, there is little data about the regression of cirrhosis-related complication, such as esophageal varices and splenomegaly, in patients with HCV-related liver cirrhosis who are treated with interferon-alpha and ribavirin combination therapy. We observed two patients with HCV liver cirrhosis, who achieved SVR after treatment with interferon-alpha and ribavirin combination, go into complete remission of esophageal varices and splenomegaly.
To our knowledge, this is the first report to demonstrate that cirrhosis-related complications, such as esophageal varices and splenomegaly, can regress after antiviral therapy in patients with HCV-related liver cirrhosis.
CASE REPORT
Case 1
A 57-year-old woman visited our outpatient clinic with positive anti-HCV test results on routine medical check-up. Initial laboratory data were as follows: white blood cell count of 3,800/μL (N:4,000-10,000), hemoglobin of 11.8 g/dL (N: 13-17), platelet count of 72,000/μL (N:150,000-450,000), creatinine of 0.7 mg/dL (0.9-1.3), aspartate aminotransferase (AST) level of 21 IU/L (N:8-38), alanine aminotransferase (ALT) level of 15 IU/L (N:4-44), total bilirubin of 0.4 mg/dL (N:0.2-1.2), albumin of 4.3 g/dL (N:3.8-5.3), and prothrombin time of 1.06 international normalization ratio (INR) (N:0.88-1.2). The HBsAg and anti-HBs were negative, but anti-HCV was positive. The level of HCV RNA was 1.32×105 copies/mL and HCV genotype was 2a.
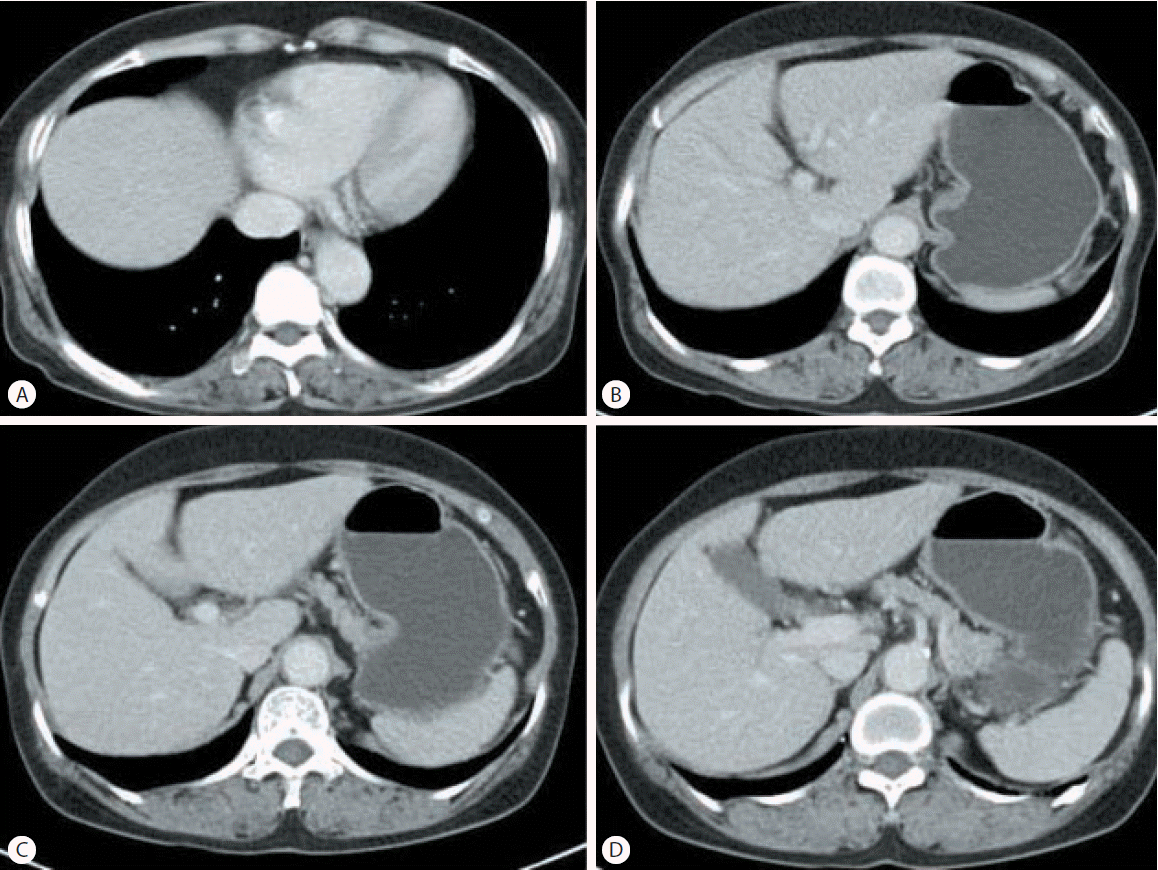
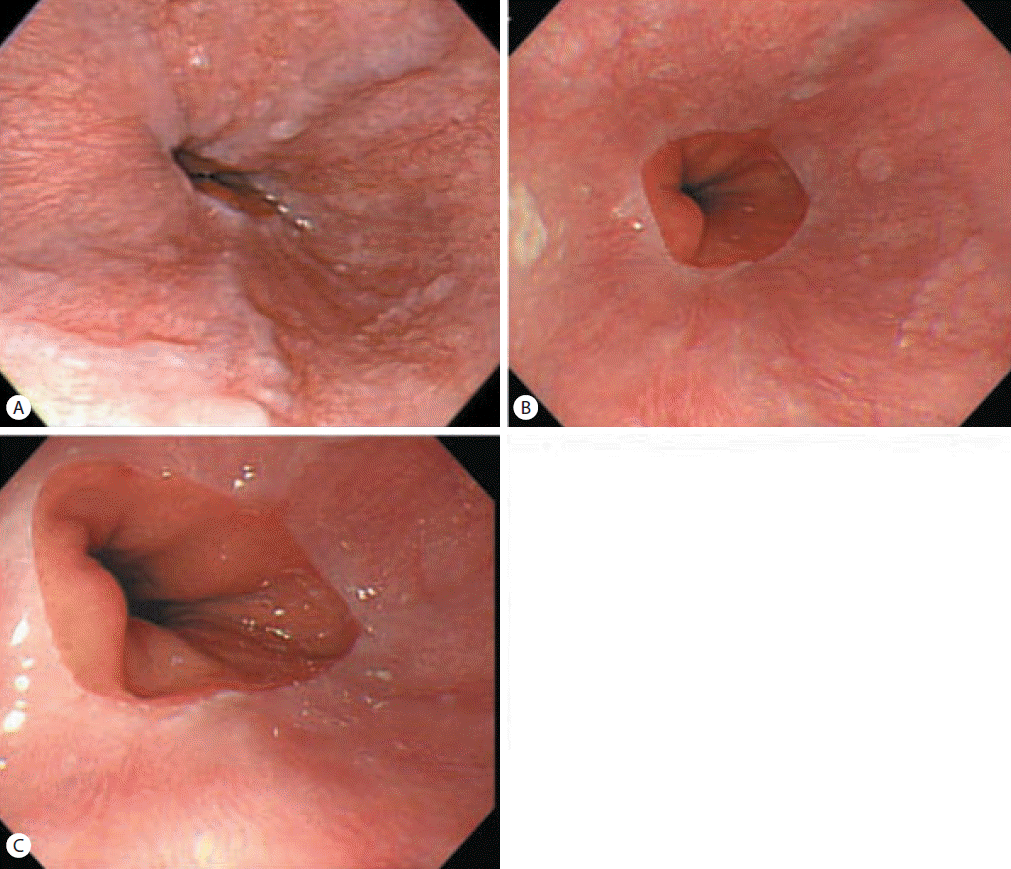
Abdominal ultrasonography showed coarse echogenicity of the liver parenchyma and splenomegaly (long axis of spleen, 11.8 cm). (Fig. 1A) Esophagogastroduodenoscopy showed presence of minimal to F1 esophageal varices on the lower esophagus and portal hypertensive gastropathy (Fig. 2A). Based on these results, the patient was diagnosed with HCV-related liver cirrhosis. Interferon-alpha 2b and ribavirin combination therapy commenced from June 30, 2004 for 24 weeks. The patient achieved SVR. HCV RNA was negative, and normal ALT persisted during follow-up. After 1 year of SVR, esophageal varices progressed to F1-F2 (Fig. 2B). After 2 years of SVR, esophageal varices decreased to minimal varices, and regressed completely (Fig. 2C, 2D) after 3 years of SVR. Abdominal computed tomography taken after 9 years of SVR showed no evidence of esophageal, para-esophageal varices, or portosytemic shunt (Fig. 3). The spleen size increased to 12.2 cm after 1 years of SVR (Fig 1B), however, its size decreased to 10.9 cm after 2 year of SVR (Fig. 1C), and decreased further to 8.9 cm (Fig. 1D) after 3 years of SVR. WBC and platelet counts increased to 5,200/μL and 171,000/μL at last follow up, respectively. Last examination was performed on August 14, 2014. There was no evidence of esophageal varices, splenomegaly or thrombocytopenia.

Abdominal ultrasonographic findings in case 1. Initial abdominal ultrasonography revealed chronic liver disease with mild splenomegaly (11.8 cm) (A). The splenomegaly had progressed to 12.2 cm after 1 years of a sustained virologic response (SVR) (B). The spleen size subsequently decreased to 10.9 cm after 2 years of an SVR (C) and to 8.9 cm after 3 years of an SVR (D).

Esophagogastroduodenoscopic findings in case 1. Minimal to F1 esophageal varices in the lower esophagus were observed prior to applying antiviral therapy (A). Esophageal varices had progressed to F1/F2 after 1 year of an SVR (B), and then decreased to minimal or F1 after 2 years of an SVR (C) and regressed completely after 3 years of an SVR (D).
Case 2
A 60-year-old man diagnosed with CHC was referred to the outpatient clinic for antiviral therapy in January 13, 2004. Initial laboratory data were as follows: WBC of 6,200/μL, hemoglobin of 16.0 g/dL, platelet count of 106,300/μL, creatinine of 0.9 mg/dL, AST level of 182 IU/L, ALT level of 238 IU/mL, total bilirubin level of 1.1 mg/dL, albumin level of 4.2 g/dL, and prothrombin time of 1.07 INR. The HBsAg and anti-HBs were negative, but anti-HCV was positive. The level of HCV RNA was 2.26×105 copies/ml and HCV genotype was 2a.
Esophagogastroduodenoscopy revealed presence of F1 esophageal varices on the lower esophagus and portal hypertensive gastropathy (Fig. 4A). Abdominal ultrasonography showed coarse echogenicity of the liver parenchyma and mild splenomegaly (long axis of spleen, 10.2 cm) [7]. He was treated with interferon alpha and ribavirin combination for 24 weeks and achieved SVR. During follow up, esophageal varices decreased in size (Fig. 4B) and completely regressed after 8 years of SVR (Fig. 4C). The spleen size decreased to 8.9 cm and platelet counts increased to 256,000/μL after 8 years of SVR.
DISCUSSION
Esophageal varices are one of the major complications in patients with liver cirrhosis. Prevalence of esophageal varices is 40% in patients with Child-Pugh class A, and up to 80% in Child-Pugh class C. It is well known that variceal bleeding is a fatal complication in patients with liver cirrhosis [8].
Histological changes in liver cirrhosis have been believed to be irreversible for a long time [9]. However, recent studies showed variable degrees of regression of liver cirrhosis in patients who achieved SVR after antiviral therapy [4-6]. Recent meta-analysis also showed that 73 of 137 patients (53%) who achieved SVR had regression of liver cirrhosis [4]. In addition, Bruno et al. demonstrated that the achievement of SVR prevents the development of esophageal varices in patients with compensated HCV-related liver cirrhosis [10]. Therefore, it is now clear that the liver cirrhosis is not a static event but a dynamic and regulated process that is reversible if the underlying cause of liver injury is eliminated [11].
Nevertheless, little data are available if complications of cirrhosis, such as esophageal varices and splenomegaly, can regress after antiviral therapy. Some studies reported spontaneous regression of esophageal varices in patient with HBsAg loss or abstinence from alcohol drinking [12,13], and Koga et al. reported 3 cases whose esophageal varices regressed after lamivudine therapy [14]. However, there was no report to demonstrate regression of esophageal varices and splenomegaly after antiviral therapy in patients with HCV-related liver cirrhosis.
Previous studies suggest that regression of liver cirrhosis usually occurred after 3 years of anti-viral therapy [4]. In our study, regression of esophageal varices occurred after 3 years and 8 years of SVR in each patient. This phenomenon might be attributable to lowering portal venous pressure following improvement of fibrosis or cirrhosis after SVR.
To our knowledge, this is the first report demonstrating that complication of liver cirrhosis, such as esophageal varices and splenomegaly, can be reversed after sustained eradication of HCV replication.
Notes
Conflicts of Interest: The authors have no conflicts to disclose.
Abbreviations
ALT
alanine aminotransferase
AST
aspartate aminotransferase
CHC
chronic hepatitis C
HCV
hepatitis C virus
SVR
sustained virologic response