Changing from NAFLD to MASLD: Similar cumulative incidence of reflux esophagitis between NAFLD and MASLD
Article information
Dear Editor,
We read the multi-society statement regarding the updated fatty liver disease nomenclature published by the nonalcoholic fatty liver disease (NAFLD) Nomenclature Consensus Group [1] with great interest. NAFLD is now termed metabolic dysfunction-associated steatotic liver disease (MASLD).
Reflux esophagitis is a common gastrointestinal ailment that affects 10–20% of the general population, and its prevalence continues to increase [2]. Major complications of reflux esophagitis include esophageal stricture, esophageal bleeding, Barrett’s esophagus, and esophageal adenocarcinoma. Moreover, reflux esophagitis is associated with anxiety, depression [3], and impaired patient-reported outcomes [4].
Reflux esophagitis is often associated with aging, hiatal hernia, and lifestyle-related factors, such as poor eating and exercise habits, and metabolic dysfunction [5,6]. Furthermore, a recent study reported that NAFLD increases the risk of reflux esophagitis [7,8].
Hagström et al. [9] previously reported that >99.5% of patients with NAFLD meet the MASLD criteria and that there is no statistical difference in the rate or risk of overall mortality or liver-related outcomes. In addition, Yoon and Jun [10] found that MASLD criteria encompass almost the entire previous NAFLD patient population (>95%), allowing seamless continuation of ongoing clinical trials for NAFLD drugs. Furthermore, Kim et al. [11] emphasized the significance of preserving and building upon existing NAFLD research to avoid unnecessary waste of research resources. However, no studies have examined the association between NAFLD and MASLD during the development of reflux esophagitis.
We aimed to compare the incidence of reflux esophagitis between patients with NAFLD and those with MASLD. A total of 18,958 consecutive patients who underwent routine health checks were evaluated using esophagogastroduodenoscopy and abdominal ultrasonography at three institutions in Japan between May 2008 and January 2021. All patients were of Asian origin. Patients who were diagnosed with reflux esophagitis using esophagogastroduodenoscopy at their initial health checkup (n=1,825), lacked data regarding alcohol consumption (n=779), reported the use of proton pump inhibitors at their initial health checkup (n=423), or had no follow-up esophagogastroduodenoscopy (n=8,315) were excluded. The data of 7,616 patients (58% male; median age 49 years [interquartile range, IQR: 41–57 years], median body mass index: 22.6 kg/m2 [IQR: 20.5–24.8 kg/m2]) were analyzed.
The diagnosis of steatotic liver disease (SLD) was based on the presence of moderate or severe hepatic steatosis on ultrasound. Esophagogastroduodenoscopy was performed after overnight fasting by endoscopists with experience performing >1,000 procedures. GIF-H260, GIF-H260Z, GIF-H290, GIF-H290Z, and GIF-XP290N endoscopes were used (Olympus, Tokyo, Japan). The endoscopy findings followed the modified version of the Los Angeles classification to describe the different grades of esophagitis severity (including grades M and A–D) based on the extent of the esophageal lesions [12]. The development of reflux esophagitis during the follow-up period was the primary outcome.
NAFLD was diagnosed in 1,458 patients, including 214 who did not fulfill the metabolic criteria for MASLD. All 214 individuals were classified as having cryptogenic SLD according to the new nomenclature. As a result, 85.4% of patients with NAFLD were also diagnosed with MASLD. The prevalence of hiatal hernia (18.0% and 18.4%), eating habits (eating within two hours before bedtime at least three times/week; 21.1% and 21.1%, respectively), and exercise habits (at least 30 min/session; 18.0% and 18.4%, respectively) were similar between the NAFLD and MASLD groups. Among the five metabolic risk factors listed in the criteria for MASLD, overweight/obesity or central obesity and low HDL cholesterol were the most and least prevalent risk factors, respectively (Supplementary Table 1).
Differences in the cumulative incidence of reflux esophagitis between the non-SLD and NAFLD/MASLD groups were analyzed using log-rank tests. The cumulative incidence of reflux esophagitis was significantly higher in the NAFLD/MASLD group than in the non-SLD group (Fig. 1). However, the cumulative incidence of reflux esophagitis was not significantly different between the NAFLD and MASLD groups. The five-year cumulative incidence of reflux esophagitis was 22.1% in patients with NAFLD and 22.6% in patients with MASLD. These results indicate that excluding cryptogenic SLD from NAFLD did not alter the cumulative incidence of reflux esophagitis. In addition, there was no significant difference in the risk of reflux esophagitis between patients with NAFLD and those with MASLD.
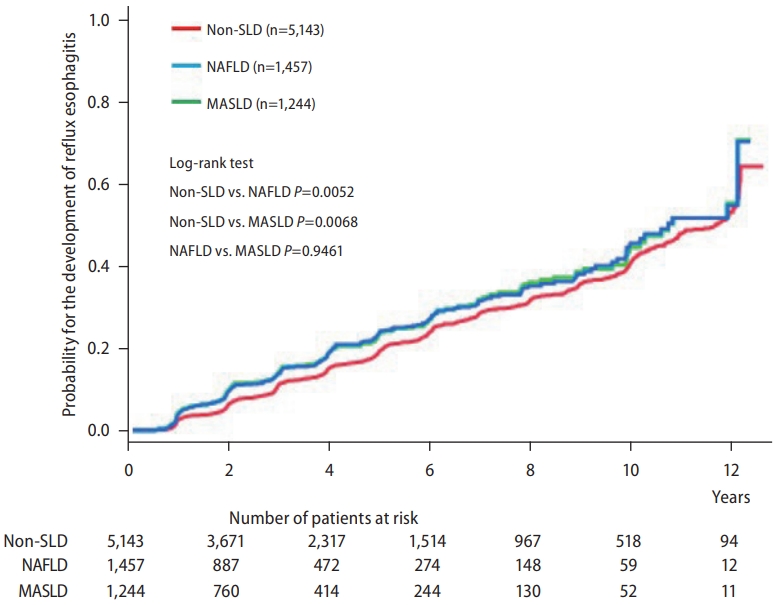
Cumulative incidence of reflux esophagitis in the non-SLD, NAFLD, and MASLD groups. SLD, steatotic liver disease; NAFLD, nonalcoholic fatty liver disease; MASLD, metabolic dysfunction-associated steatotic liver disease.
Therefore, the MASLD diagnostic criteria may be useful for the efficient screening of patients at high risk of developing reflux esophagitis.
Notes
Authors’ contribution
Shuhei Fukunaga: study concept, design, and statistical analysis; Michita Mukasa: data extraction, interpretation of data, and critical revision of the manuscript; Dan Nakano: interpretation of data, drafting, and critical revision of the manuscript; Tsubasa Tsutsumi: interpretation of data and critical revision of the manuscript; Takumi Kawaguchi: interpretation of data and critical revision of the manuscript.
Conflicts of Interest
Takumi Kawaguchi received lecture fees from Janssen Pharmaceutical K.K., Taisho Pharmaceutical Co., Ltd., Kowa Company, Ltd., Otsuka Pharmaceutical Co., Ltd., Eisai Co., Ltd., ASKA Pharmaceutical Co., Ltd., AbbVie GK., and EA Pharma Co., Ltd. The other authors have no conflicts of interest.
Acknowledgements
This research was supported by the Japan Agency for Medical Research and Development (AMED) under grant number JP23fk0210090.
Abbreviations
SLD
steatotic liver disease
NAFLD
nonalcoholic fatty liver disease
MASLD
metabolic dysfunction-associated steatotic liver disease
SUPPLEMENTAL MATERIAL
Supplementary material is available at Clinical and Molecular Hepatology website (http://www.e-cmh.org).
Baseline characteristics and outcomes of patients with NAFLD and MAFLD, respectively
