Loco-regional therapies competing with radiofrequency ablation in potential indications for hepatocellular carcinoma: a network meta-analysis
Article information
Abstract
Background/Aims
There is no clear consensus on the relative ranking of interventional and radiation techniques with indications similar to those of radiofrequency ablation (RFA) for the treatment of early hepatocellular carcinoma (HCC). We used a network meta-analysis to compare the efficacy of non-surgical treatments for early HCC.
Methods
We searched databases for randomized trials assessing the efficacy of loco-regional treatments for HCCs ≤5 cm with no extrahepatic spread or portal invasion. The primary outcome was the pooled hazard ratio (HR) for overall survival (OS), and secondary outcomes included overall and local progression-free survival (PFS). A frequentist network meta-analysis was performed, and the relative ranking of therapies was assessed with P-scores.
Results
Nineteen studies comparing 11 different strategies in 2,793 patients were included. Chemoembolization plus RFA improved OS better than RFA alone (HR 0.52, 95% confidence interval [CI] 0.33–0.82; P-score=0.951). Cryoablation, microwave ablation, laser ablation, and proton beam therapy had similar effects on OS compared with RFA. For overall PFS, but not local PFS, only chemoembolization plus RFA performed significantly better than RFA (HR 0.61, 95% CI 0.42–0.88; P-score=0.964). Injection of percutaneous ethanol or acetic acid was significantly less effective than RFA for all measured outcomes, while no differences in progression outcomes were identified for other therapies included in the network.
Conclusions
Our results suggest that chemoembolization combined with RFA is the best option for local treatment of early HCC. Cases with potential contraindications for RFA may benefit from a tailored approach using thermal or radiation modalities.
Graphical Abstract
INTRODUCTION
Surgical resection and liver transplantation are the mainstays of curative treatment for early hepatocellular carcinoma (HCC) within the Milan criteria for tumor size and number [1,2]. Radiofrequency ablation (RFA) has achieved comparable outcomes to surgery, with fewer complications, in several randomized controlled trials (RCTs) and remains the standard of care for patients with small HCCs that are unresectable or medically inoperable, or for whom a donor is not available [3,4]. However, despite advances in imaging guidance and efforts to improve procedural safety [5], poor accessibility to intrahepatic lesions as well as proximity to blood vessels or biliary ductslimit the broad application of RFA [6,7].
Since the initial clinical implementation of RFA, prospective and retrospective studies on other interventional and radiation techniques have reported favorable outcomes in terms of local tumor progression and life expectancy. Several milestone trials of loco-regional methods in early HCC have focused on alternative primary strategies such as microwave ablation (MWA), cryoablation (CA), stereotactic body radiation therapy (SBRT), and proton beam therapy (PBT). These approaches, alone or combined with transarterial chemoembolization (TACE), are considered the next standard option for tumors within the Milan criteria according to international guidelines [1,8-12].
There is no consensus on the relative effectiveness of these local treatments because there is a lack of high-level direct evidence from head-to-head pairwise comparisons. Moreover, previous meta-analyses did not consider all relevant interventions, including radiotherapies, that had comparable efficacies, as demonstrated in randomized or matched-pair studies, and that were useful alternativesto standard therapy in HCC indicated for RFA [13,14]. Before the results of pairwise treatments become available, there is an urgent need for evidence-based indicators of the most appropriate procedure for early unresectable tumors. To address this critical real-life question, we conducted a systemic literature review of prospective pairwise comparisons of different types of loco-regional HCC treatments. Based on our findings, we performed a network meta-analysis (NMA) comparing treatment efficacy in terms of controlling target lesions and improving patient survival and then estimated the relative benefits of individual approaches by combining direct and indirect evidence.
MATERIALS AND METHODS
This study adhered to the standard guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension statement for network meta-analysis (PRISMANMA) [15], and the study was registered in PROSPERO (protocol no. CRD42021278742). The institutional review board of Asan Medical Center approved this trial-level NMA, and the requirement for informed consent of individual patients was waived (IRB No. 2021-0823). Data generated or analyzed during the study are available from the corresponding authors by request.
Eligibility criteria
RCTs published as full-text articles in peer-reviewed journals that prospectively examined the efficacy of loco-regional therapies for primary or recurrent HCCs ≤5 cm without extrahepatic spread or portal invasion, which are generally accepted as standard indications for RFA, were eligible for this NMA [11,16]. We included studies comparing single or combined modalities with alternative intervention(s) that reported extractable data for at least one measure of overall survival (OS) and local or overall recurrence/progression. Only full text articles published in English in peer-reviewed journals were included.
Studies that had any arm of liver resection or transplantation or those that included any HCC >5 cm in diameter, which can be difficult to ablate, were excluded from the analysis. Duplicates, letters, conference proceedings, meeting abstracts, and prospective or retrospective studies of non-randomized design were also excluded.
Search strategy and selection criteria
Two separate literature searches were conducted to identify studies relevant to this NMA. The primary search was conducted on March 17, 2021, using the following databases: PubMed, EMBASE, Cochrane Library, CINAHL, and Web of Science. The search was limited to articles published in English between January 1, 2000, and March 17, 2021. The updated search was conducted on February 17, 2023, and used the same search strategy and databases to identify any newly published studies that met the inclusion criteria. Furthermore, we checked references cited in the relevant systematic reviews and meta-analyses. The detailed search strategy with query terms is presented in Supplementary Table 1. Two reviewers (JA and HIK, with 12 and 6 years of experience in the field of HCC treatment, respectively) independently screened all titles and abstracts identified by the searches and then scrutinized all the full manuscripts containing potentially relevant studies selected by either reviewer using the predefined criteria. Any discrepancies were resolved by discussion and consensus, or with the participation of an additional reviewer (JHS, with 19 years of experience in the field of HCC treatment).
Outcome definition and data extraction
The primary outcome was OS defined as the time from the date of enrollment to death from any cause. Secondary outcomes were overall progression-free survival (PFS) and local PFS. Based on the definition of endpoints used in all the included trials, overall progression included the following local and distant tumor events: (1) local progression defined as intrahepatic tumor recurrence or progression onto or along the peripheral margin of the treated lesion; and (2) development of any new HCC remote from the treated site, in any location, defined as distant progression. Overall PFS was measured by the interval from enrollment to either local/distant progression or death, whichever was first. For studies in which PFS was not reported as an endpoint, time elapsed to objective tumor progression or recurrence (TTP) was substituted asthe secondary outcome in the NMA [17]. The principal data extracted or derived from the included studies were the log hazard ratio (HR) and standard error (SE) or relevant information allowing estimation of HR and SE (e.g., an HR and confidence interval [CI] or P-value for survival, recurrence, and/or progression). All data were publicly available or computable from the individual studies. Additional summary data including details ofstudy design, treatment methods, and numbers of patients and their demographics were also extracted (Table 1).
Risk of bias assessment
Risk of bias was assessed for each study independently by two of the authors (JA and HIK) using the revised Cochrane risk-of-bias tool for RCTs [18].
Statistical analysis
To conduct the NMA, we required the log HRs for the survival outcomes and the corresponding SEs. However, for studies reporting only the P-value for the log-rank test along with the total number of events (e.g., deaths or progressions) for comparison groups, we derived the log HRs and SEs indirectly from the log-rank test results. For studies with no available log-rank test or log HRs, we calculated the log HRs and SEs indirectly from the Kaplan–Meier curves after constructing >15 time intervals [19]. After obtaining the summary statistics for the survival outcomes, we derived the therapeutic hierarchy for several therapies from an NMA in which we treated RFA as the control group.
We initially fitted both the fixed effects and random effects models simultaneously and evaluated study heterogeneity across the included trials. Statistical heterogeneity was calculated using the Higgins and Thomson I2 statistic, which describes the percentage of total variation across studies due to heterogeneity rather than chance [20]. We defined the substantial heterogeneity range as comprising values >50%. We also considered Cochran’s Q test for heterogeneity. Since there was no substantial evidence for heterogeneity across the studies with respect to any endpoint, we reported the results for the fixed effect model only. For direct and indirect comparisons between interventional and radiation techniques, we used a frequentist NMA approach employing weighted least squares regression [21]. In order to rank the treatments in terms of superiority, we computed the P-score for each treatment, which measures the degree of certainty that the treatment is better than another treatment, averaged over all the competing treatments [22]. We also performed subgroup analyses for the sub-networks involving studies based on HCC nodules ≤3 cm. Details of the statistical methods are provided in the Supplementary methods. All data analyses were performed using R packages (version 4.0.4.) netmeta and gemtc to conduct the main NMA and network-meta regression.
RESULTS
Study selection and characteristics
Figure 1 shows a flowchart of the study selection process. A total of 4,209 titles and abstracts of potentially relevant studies were screened. Of these, 48 fulfilled the eligibility criteria for full-text assessment. We examined the references in the relevant systematic reviews but identified no new records, as all references were already included in our database search results. Of the 48 studies, 29 were excluded after applying the exclusion criteria (Supplementary Table 2). Finally, 19 RCTs investigating 11 interventions in 2,793 patients were used for the NMA; these included the following treatment arms: RFA (1,124 patients in 15 trials) [6,8,10,23-34], TACE+RFA (115 in 2) [31,32], MWA (276 in 4) [23,25,34,35], CA (180 in 1) [10], laser ablation (LA; 70 in 1) [26], PBT (72 in 1) [8], TACE (84 in 1) [35], TACE+MWA (89 in 1) [35], percutaneous ethanol (PEI; 585 in 9) [6,24,27-29,33,36-38] and acetic acid injections (PAI; 159 in 3) [29,30,38], and TACE+PEI (39 in 2) [36,37].

PRISMA flow diagram of the process of screening and selecting studies. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, randomized controlled trial; HCC, hepatocellular carcinoma.
A network map of the treatment relationships and comparisons is presented in Figure 2. Node size and line thickness were proportional to the number of included patients and number of trials, respectively. Characteristics of the 19 studies included in the network are summarized in Table 1. The percentage of patients with solitary HCC ranged from 56.9% to 100%, while the percentage of patients with multifocal HCCs ranged from 0% to 43.1%. The number of tumors per patient in each study ranged from 1 to 4, and only eight patients in two trials, Shiina et al. [33], 2005 and Paul et al. [30], 2020, had >3 index lesions. Overall, 37.9% to 97.2% of patients were classified as Child-Turcotte-Pugh (CTP) class A, and 2.8% to 55.9% of patients were classified as CTP class B. All included trials reported OS as a study outcome; 11 included overall PFS (or TTP); and 11 included local PFS (or TTP). Local or overall PFS results were substituted for the corresponding TTP in seven and one studies, respectively.
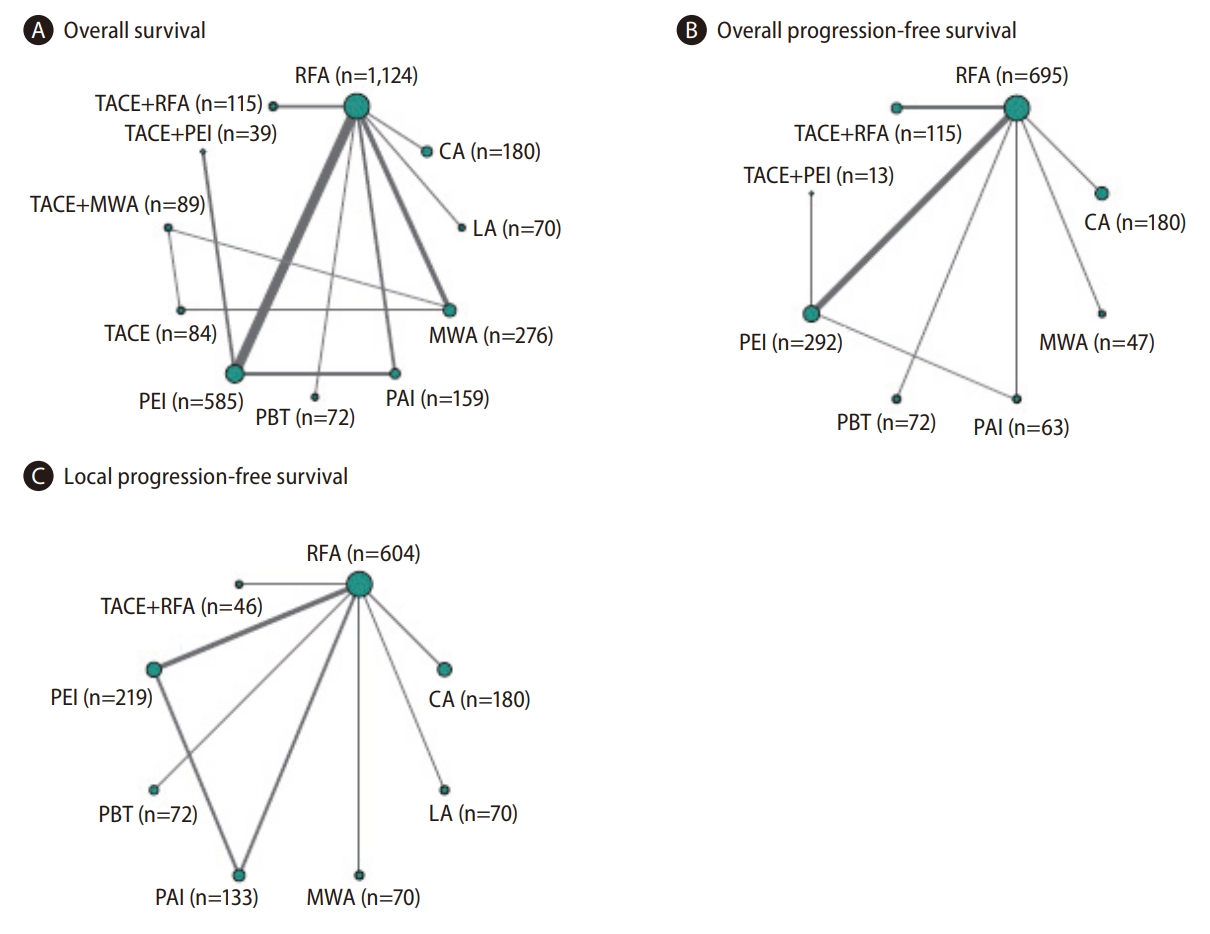
Network plots for (A) overallsurvival, (B) overall progression-free survival, and (C) local progression-free survival for direct comparisons of 19, 10, and 9 selected RCTs, respectively. Circle sizes reflect numbers of participants, while line widths reflect numbers of direct comparisons. The absence of a connecting line between two treatments indicates that there was no direct comparison. RCT, randomized controlled trial; RFA, radiofrequency ablation; TACE, transarterial chemoembolization; PEI, percutaneous ethanol injection; MWA, microwave ablation; PBT, proton beam therapy; PAI, percutaneous acetic acid injection; LA, laser ablation; CA, cryoablation.
Risk of bias assessments
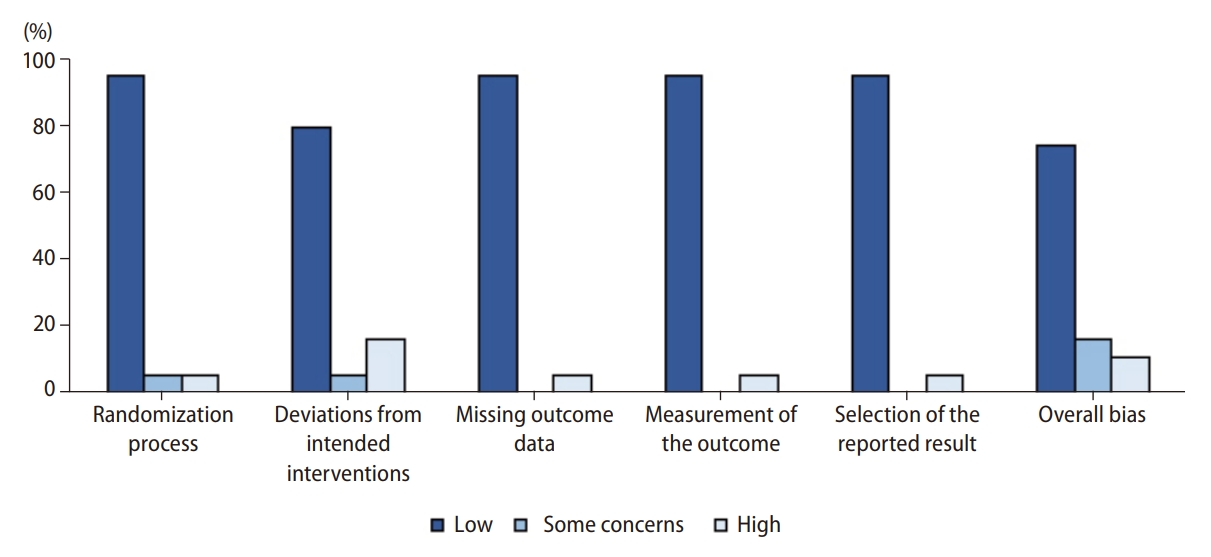
Most studies were graded as low risk for the domains including randomization process, missing outcome data, measurement of outcome data, and selection of the reported result. Three studies reported the results of a modified intention-to-treat analysis, which were considered to be at high risk for domain deviations from intended interventions. Overall, the trials were considered to be at low risk for bias. Details of these assessments are presented in Figure 3 and Supplementary Figure 1.
OS analysis
The NMA included all RCTs in the OS analysis (Fig. 2A). Only TACE+RFA had significantly better OS than RFA when used for HCCs ≤5 cm (HR, 0.52; 95% CI, 0.33–0.82; Fig. 4A). Conversely, PEI (1.51, 1.16–1.96) and PAI (1.99, 1.30–3.06) resulted in poorer OS than RFA. No significant differences in OS were observed between RFA and the following treatments: TACE+MWA, CA, PBT, MWA, and LA. Probabilistic ranking metrics based on P-scores indicated that TACE+RFA (0.951) also ranked highest of all 11 treatment classes with respect to magnitude of treatment effect on OS, with a 61.4% probability of being the most effective option on the rankogram (Fig. 4A and Supplementary Fig. 2A). TACE+MWA and CA were the second-best and third-best treatments, respectively, and PAI had the highest probability (48.3%) of being the lowest ranked. Compared to RFA, no significant difference in OS was found with TACE (HR, 1.53; 95% CI, 0.74–3.16) despite having the second lowest P-score (0.279). The same trend in the ranking of treatments according to OS was observed for the extrapolated parametric survival NMA model for time-to-event (Fig. 5).
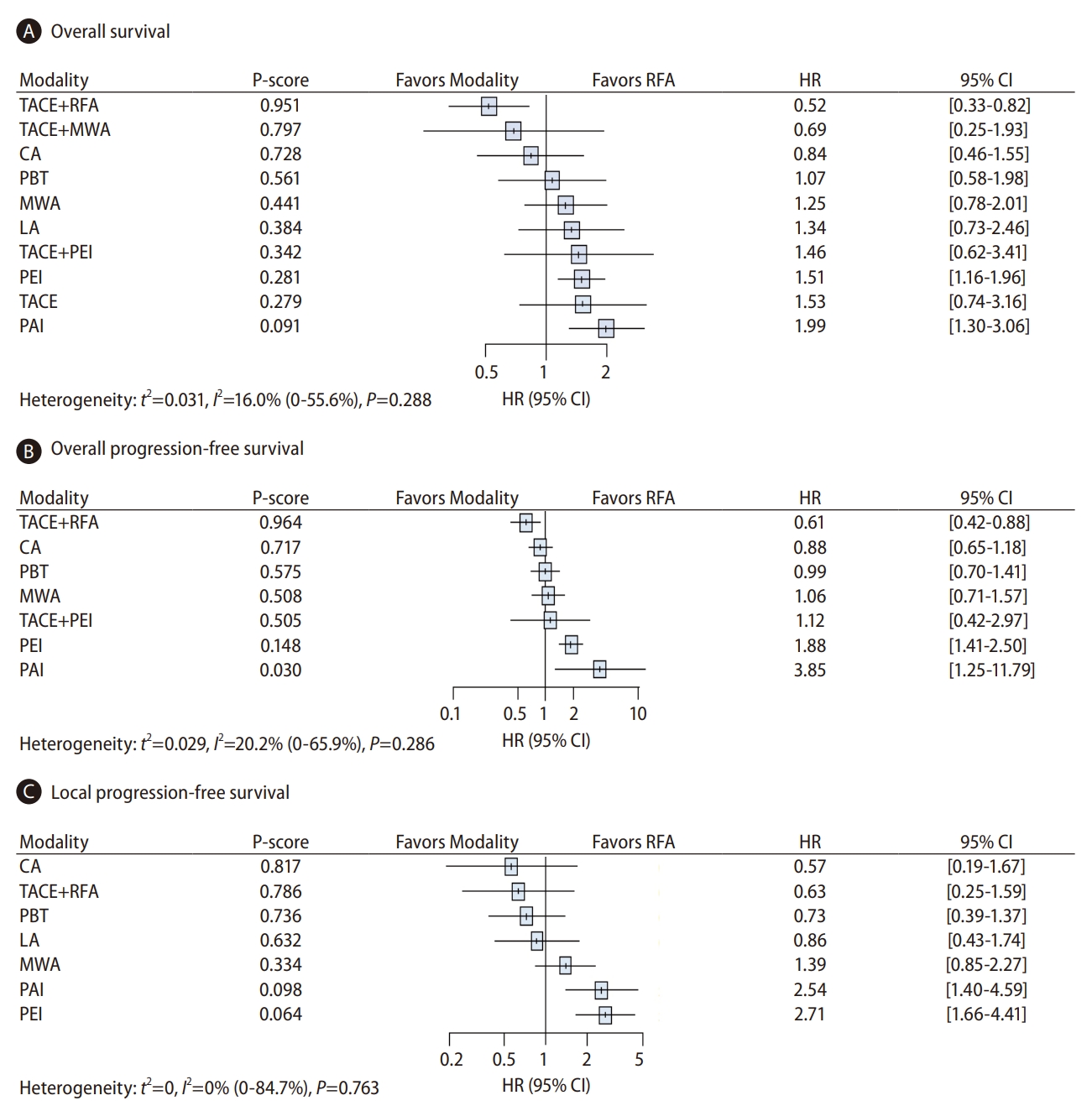
Forest plots of the fixed effects network meta-analysis modelsfor (A) overallsurvival (OS), (B) overall progression-free survival (PFS), and (C) local PFS, with treatments ranked by the probability of being the best therapy based on P-scores in the trials finally selected. (A) The OS model. Data used were from all 19 trialsinvestigating 11 treatment modalitiesinvolving the 2,793 patients that were finally selected for this study. (B) The overall PFS model. Data used were from 10 trials investigating eight treatment modalities in 1,477 patients. (C) The local PFS model. Data used were from nine trials investigating eight treatment modalities in 1,394 patients. HR, hazard ratio; CI, confidence interval; TACE, transarterial chemoembolization; RFA, radiofrequency ablation; MWA, microwave ablation; CA, cryoablation; PBT, proton beam therapy; LA, laser ablation; PEI, percutaneous ethanol injection; PAI, percutaneous acetic acid injection.

Overall survival probabilities with time for each treatment, based on estimates of the relative treatment effects. TACE, transarterial chemoembolization; RFA, radiofrequency ablation; MWA, microwave ablation; CA, cryoablation; PBT, proton beam therapy; LA, laser ablation; PEI, percutaneous ethanol injection; PAI, percutaneous acetic acid injection.
In pairwise comparisons within the complete network of 19 trials, TACE+RFA was significantly superior with respect to OS to every other treatment except TACE+MWA, CA, and PBT (Fig. 6). In addition, the four thermal or radiation therapies (i.e., CA, RFA, PBT, and MWA) were associated with significantly greater OS than PEI or PAI.

League table showing hazard ratios(HRs) for pairwise comparisons of overallsurvival (OS) and overall progression-free survival (PFS) between treatments. Comparisons should be read from left to right. HRs(95% confidence intervals[CI]) for comparisons are in the cells shared by the column-defining and row-defining interventions. Numbers written in bold are statistically significant. For OS, an HR of <1 favors the row-defining treatment. For overall PFS, an HR of <1 favors the column-defining treatment. NA, not applicable; TACE, transarterial chemoembolization; RFA, radiofrequency ablation; MWA, microwave ablation; CA, cryoablation; PBT, proton beam therapy; LA, laser ablation; PEI, percutaneous ethanol injection; PAI, percutaneous acetic acid injection.
Overall PFS analysis
To evaluate overall PFS, we analyzed the relevant data from 10 studies involving eight different therapies in 1,477 patients (Fig. 2B). Compared to RFA alone, significant HRs for overall PFS were found for TACE+RFA (HR, 0.61; 95% CI, 0.42–0.88) and PAI (3.85; 1.25–11.79) (Fig. 4B). These two treatments were ranked in first and last place, respectively, based on P-score (0.964 and 0.030, respectively). The ranking profile was generally consistent with that of OS (Supplementary Fig. 2B). In addition, the seven pairwise treatment comparisons indicated a significant advantage of TACE+RFA over RFA, MWA, PEI, or PAI in improving overall PFS (Fig. 6).
Local PFS analysis
Local PFS analysis comprised nine studies investigating eight therapeutic options in 1,394 patients (Fig. 2C). No treatment modalities detected a local PFS benefit when compared with RFA (Fig. 4C). When ordered by P-score, CA and TACE+RFA were ranked in first and second place, respectively. The rankogram also indicated that CA was the most effective treatment in prolonging local PFS duration (Supplementary Fig. 2C). In terms of pairwise comparisons, all treatment pairs other than PAI and PEI yielded comparable rates of local PFS (Supplementary Fig. 3).
Adverse events
A total of 16 studies reported major adverse events among 2,304 patients undergoing 11 distinct therapies [6,8,10,23-31,33-38]. Due to the lack of a consistent definition for major adverse events across the studies, a quantitative assessment could not be made. The observed prevalence of major complications ranged from 0% to 7.7%,depending on the specific criteria used in each study.
Regarding treatment modalities, RFA was associated with a major complication rate that varied from 0% to 6.9% across 12 studies. Post-RFA complications included lung-related problems such as hemothorax, pneumothorax, and pleural effusion, in addition to hemorrhage, hepatic decompensation (manifesting as ascites or jaundice),malignant cell seeding, infection, and hepatic infarct/necrosis.
The major complication rates for other treatment modalities were as follows: PEI, 0–2.9% (eight studies); PAI, 0–7.7% (three studies); MWA, 2.2–2.8% (two studies); and TACE+RFA, 2.2–2.9% (two studies).Notable adverse events associated by modality were as follows: PEI (one death due to bowel infarction, neoplastic seeding, and liver abscess), PAI (two deaths resulting from hepatic necrosis and tumor rupture, tumor seeding, and gross hematuria), MWA (hemorrhage and tumor seeding), and TACE+RFA (hepatic decompensation and segmental hepatic infarction). The remaining six treatment modalities were each investigated in a single study. A comprehensive description of adverse events is presented in Supplementary Table 3.
Subgroup analysis
Among the 19 RCTs, seven studies included a total of 1,128 patients with HCC nodules ≤3 cm in diameter undergoing six distinct therapies. These studies were included in a network created to analyze HRs for OS. No significant differences in OS (HR [95% CI], 0.67 [0.21–2.20]; Supplementary Fig. 4A) were found between the combined TACE+RFA and RFA alone groups, although survival was slightly better in the TACE+RFA group. Similar results were obtained for both overall PFS in four studies with 652 patients (0.84 [0.51–1.41]) and local PFS in three studies with 420 patients (0.63 [0.25–1.59]), as shown in Supplementary Figure 4B and 4C.
Assessment of transitivity and inconsistency
Overall, the transitivity assumption was not challenged, as there were no significant differences in the baseline parameters examined to evaluate its plausibility (Supplementary Fig. 5). There were also no significant differences for either OS or local PFS in terms of inconsistencies between direct and indirect estimates in the node-splitting analysis within the closed loop in the evidence network (PEI-PAI-RFA) (Supplementary Table 4).
DISCUSSION
The above analysis based on the published outcomes of RCTs of loco-regional therapies revealed that combined TACE and RFA occupies the first position in a ranking of non-surgical treatments of early HCCs meeting the Milan criteria. The analysis based on the integrated scores for OS and PFS showed that combined TACE and RFA is superior to all the other mono- or dual-therapeutic options, including TACE and RFA individually. In addition, all single interventions, other than PEI and PAI, the oldest techniques for the treatment of HCC, had comparable survival- and progression-related efficacy compared to RFA, the standard strategy according to the current evidence-based management of this disease stage.
The RCTs and meta-analyses had limitations in terms of sample size,study quality, and statistical power. Nonetheless, the cumulative evidence from these studies indicated that TACE+RFA is superior to RFA alone in terms of survival and/or recurrence, particularly for studies including HCCs >3 cm, and that it was no associated with any significant major complications [39,40]. It is likely that the benefits of TACE followed as soon as possible by RFA derive from (1) improved control of microsatellite lesions due to the larger treated area [41]; (2) reduced vascular cooling effect that can lead to incomplete ablation; (3) prevention of portal invasion of the tumor by hepatic arterial and portal venous flows occluded by embolic materials; (4) boosting of the thermal effect by chemotherapeutic drugs and ischemic edema; and (5) optimization of heat diffusion by the disruption of intratumoral septa [42,43]. Our investigation of nodules up to 5 cm in diameter also concluded that this combination was more effective than RFA, MWA, or LA therapy alone. Furthermore, given the discrepancy between the overall (positive) and local (negative) PFS results of TACE+RFA, our results suggest that the addition of TACE may prolong OS, compared with RFA alone, by targeting microsatellites together around the target lesion. Surprisingly, our analysis revealed no beneficial effect on OS when adding TACE to MWA, although this combination was comparable to TACE+RFA based on pairwise comparisons. A retrospective study in the U.S. comparing 38 patients treated with TACE+RFA and 51 treated with TACE+MWA for HCCs of varying sizes yielded similar safety and efficacy outcomes [44]; the ranking of these combination therapies in term of safety and efficacy needs to be confirmed by further relevant RCTs.
Our findings indicate that TACE+RFA deserves primary consideration for curative control of early HCCs, apart from ultrasound-invisible HCCs requiring radiographic guidance for curative RFA treatment by TACE-induced iodized oil retention [45]. Subgroup analyses based on sub-networks of RCTs explicitly reporting data restricted to patients with HCC nodules ≤3 cm in diameter reported no significant differences in local PFS, overall PFS, or OS between combined TACE+RFA and RFA alone (Supplementary Fig. 4). The study from Japan of Shibata et al. [32] included in our network illustratesthis point. Nevertheless, these findings support the idea that RFA monotherapy is adequate for treating small (≤3 cm) HCCs, when patient convenience, hospital stay, and medical costs are taken into account [46]. Therefore, combination therapy may be more useful for medium-sized (3–5 cm) HCCs, as complete necrosis is difficult to achieve with RFA alone, particularly in infiltrating tumor types [16]. This conclusion is consistent with the current recommendations stipulated in the recently updated Korean guidelines [2].
For imaging-guided percutaneous ablation techniques, such as RFA, MWA, CA, and LA, our direct and indirect comparison data revealed no significant differences in both survival and progression endpoints among the mono-modalities; this is consistent with reports of individual paired comparative studies based on various design types [13,23,25,26]. Each technique has its own advantages and limitations compared with the traditional RFA reference: for instance, MWA can produce larger coagulation areas than RFA with shorter ablation times; it is also less sensitive to the heat-sink effect and hence achieves adequate ablation of nodules close to large vessels; however, it is contraindicated for treating nodules at high-risk locations as well as subcapsular nodules [47,48]. In spite of technical difficulties, LA is safer for treating nodules at difficult locations, as well as multiple lesions in one session, as it uses multiple fibers and spares the uninvolved hepatic parenchyma [49]. Lastly, CA, which is much less painful than RFA, has the advantage of protecting better against vascular and biliary injuries by monitoring the extent of ablation during the procedure, despite producing larger ablation volumes with multiple probes [50]. Our findings provide prognostic evidence justifying a tailored approach in which other liver-directed thermal ablation procedures are placed alongside RFA as standard-of-care for maximizing treatment efficacy and minimizing procedure-related complications in patients considered to be candidates for HCC ablation.
Several HCC studies have reported good outcomes with hypo-fractionated PBT having a finite range of energy deposition and thus low rates of hepatic and gastrointestinal toxicity relative to photon beam therapy [8,51]. A recent U.S. registry study of 918 patients with T1 or T2 HCC suggested that PBT may have superior survival outcomes compared to SBRT [52]. The latter is another conformal external technique, which was not included in our NMA because of the absence of relevant RCTs. However, a meta-analysis of 70 non-comparative observational studies suggesting that the two radiotherapies have similar efficacy with respect to OS and overall and local PFSs warrants prospective corroboration [53]. Based on our NMA findings and the existing evidence, PBT (or SBRT if PBT is not feasible) may be considered alternative treatments for percutaneous options including RFA, particularly for nodules considered to be at high risk, with limited accessibility, or invisible under ultrasound guidance [54,55]. Unlike injection therapies with ethanol or acetic acid, which have few clinical applications and have been found to be inferior to RFA in numerous comparisons [6,24,33], TACE, with a broader spectrum of indications, remains the first choice in early-stage cases that are neither suitable nor appropriate for local ablation when based on the stage migration strategy [1]. Pairwise direct and indirect evidence from our NMA may justify a second-best role of TACE monotherapy as an alternative next-line option for HCCs below the intermediate stage [35]. It should also not be overlooked that several matched and non-matched studies of within-Milan HCCs have found that TACE has comparable effects to therapeutic ablation and radiation on tumors and patients [56,57].
Apart from the inherent limitations of collecting aggregate patient data from completed studies, some limitations of this work should be acknowledged. First, we could not include an estimate of safety profile as an outcome of interest in the present NMA because of the heterogeneous criteria for, and definitions of, procedure-related adverse events across the studies. However, almost all the intervention arms in all the RCTs had <5% serious adverse events(Table 1). Second, a few patients with CTP class C liver function or >3 target lesions who are not usually good candidates for loco-regional therapies but were included in our analysis, albeit rarely, may have affected the interpretation of survival and efficacy outcomes (Table 1) [25,28,30,36]. Lastly, in most (n=12) of the included RCTs, the inclusion criteria specified HCCs up to 5 cm, which prevented us from enrolling only ideal candidates with tumors ≤ 3cm. However, we were able to determine the outcomes for HCC lesions ≤3 cm through a subgroup analysis of seven studies.
In conclusion, in this loco-regional therapy-based NMA, we found that TACE+RFA was ranked highest with regard to both survival and progression outcomes in the treatment of patients with early HCCs ≤5 cm. Equivalent outcomes are likely for HCCs within the Milan criteria that can be optimally treated by thermal ablation using a variety of possible energy sources or PBT. Further evidence concerning specific indications for the individual modalities is urgently needed to develop precisely tailored strategies.
Notes
Authors’ contribution
HI Kim, J An, and JH Shim contributed to the study concept and design, acquisition, analysis and interpretation of data, verification of the underlying data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content. S Han contributed to the statistical analysis, verification of the underlying data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content. All authors confirm that they had full access to all the data in the study and accept responsibility for submission of this manuscript.
Conflicts of Interest
The authors have no conflictsto disclose.
Acknowledgements
This study was supported by grants from the National Research Foundation of Korea funded by the Ministry of Science and ICT (NRF-2022R1A2C3008956 and RS-2022-00166674), the Research Supporting Program of The Korean Association for the Study of the Liver and The Korean Liver Foundation (KASLKLF2018-05) and Asan Institute for Life Sciences, Asan Medical Center (2022IP0046).
Supplementary materials
Supplementary material is available at Clinical and Molecular Hepatology website (http://www.e-cmh.org).
Detailed profiles of risk of biasin the randomized controlled trials.
Ranking curves displaying the probabilities per rank of (A) overall survival, (B) overall progression-free survival (PFS), and (C) local PFS for each treatment. PAI, percutaneous acetic acid injections; CA, cryoablation; RFA, radiofrequency ablation; LA, laser ablation; PBT, proton beam therapy; TACE, trans-arterial chemoembolization; MWA, microwave ablation; PEI, percutaneous ethanol injection.
League table showing hazard ratios of local progression-free survival for pairwise comparisons between treatments. Comparisons should be read from left to right. HRs (95% confidence intervals [CI]) for comparisons are in the shared cells. PAI, percutaneous acetic acid injections; CA, cryoablation; RFA, radiofrequency ablation; LA, laser ablation; PBT, proton beam therapy; TACE, trans-arterial chemoembolization; MWA, microwave ablation; PEI, percutaneous ethanol injection; HRs, hazard ratios.
Treatment effects for (A) overall survival and (B) overall and (C) local progression-free survivals in the sensitivity analysis based on randomized controlled trials(RCTs) limited for HCC nodules ≤3 cm in diameter. Six, five, and five treatmentsfrom seven, four, and three of the 19 RCTs, respectively, were included in networks created to analyze hazard ratios (HRs) for overall survival and overall and local progression-free survivals, respectively. PAI, percutaneous acetic acid injections; CA, cryoablation; RFA, radiofrequency ablation; LA, laser ablation; PBT, proton beam therapy; TACE, trans-arterial chemoembolization; MWA, microwave ablation; PEI, percutaneous ethanol injection; HCC, hepatocellular carcinoma; CI, confidence interval.
Checking the transitivity assumption. The study and population baselines evaluable in all trials were included in testing transitivity. The patients in all of the comparisons were similar in age (left) and other main parameters with a P-value>0.05 (right). On the left, the blue bar indicates mean age with 3 standard deviations of the mean, while the red bar indicates the median age with range. *Median ages with interquartile range were provided in Vietti Violi et al. [34], 2018; Shiina et al. [33], 2005 was excluded from the assessment because of the absence of data on the mean or median age of the patients. PAI, percutaneous acetic acid injections; CA, cryoablation; RFA, radiofrequency ablation; LA, laser ablation; PBT, proton beam therapy; TACE, trans-arterial chemoembolization; MWA, microwave ablation; PEI, percutaneous ethanol injection; CTP, Child-Turcotte-Pugh.
Search strategies with query terms
Exclusion criteria and characteristics of excluded studies(n=29)
Major adverse eventsin 16 randomized controlled studies
Node-splitting analysis of inconsistency between direct and indirect effectsin a closed loop network (PEI-PAI-RFA) in terms of overallsurvival and local progression-free survival
Abbreviations
CA
cryoablation
CI
confidence interval
CTP
Child-Turcotte-Pugh
HCC
hepatocellular carcinoma
HR
hazard ratio
LA
laser ablation
MWA
microwave ablation
NMA
network meta-analysis
OS
overall survival
PAI
percutaneous acetic acid injection
PBT
proton beam therapy
PEI
percutaneous ethanol injection
PFS
progression-free survival
PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
RCT
randomized controlled trial
RFA
radiofrequency ablation
SBRT
stereotactic body radiation therapy
SE
standard error
TACE
transarterial chemoembolization
TTP
time-to-progression
References
Article information Continued
Notes
Study Highlights
• In a network meta-analysis of 19 randomized trials exploring 11 loco-regional therapies, only the combination of TACE and RFA showed a significant improvement in the overall survival of patients with HCC measuring ≤5 cm, compared to RFA alone (HR, 0.52; 95% CI, 0.33–0.82). This combination treatment also ranked first based on P-score (0.964).
• An analysis of overall progression-free survival involving eight treatments in ten trials revealed that the HR for the combination therapy was significantly better than that for RFA alone (HR, 0.58; 95% CI, 0.38–0.89), again having the highest P-score (0.999).
• No modalities outperformed RFA alone in terms of local progression-free survival rates.