| Clin Mol Hepatol > Volume 27(4); 2021 > Article |
|
ABSTRACT
ACKNOWLEDGMENTS
Figure┬Ā1.
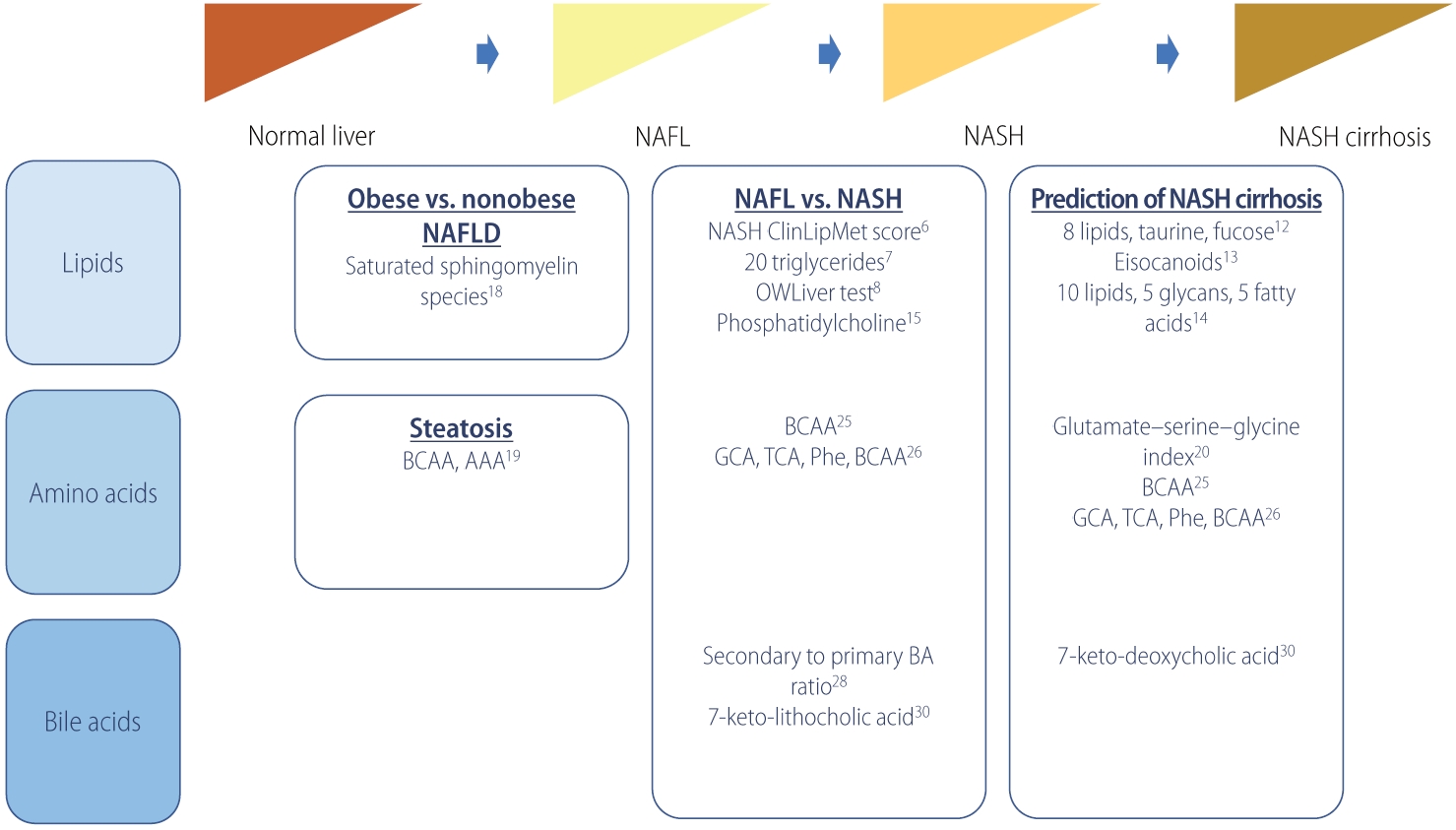
Table┬Ā1.
| Study | Outcome | No. | Metabolites (panel) | Performance | |
|---|---|---|---|---|---|
| Lipid | |||||
| ŌĆā | Zhou et al. [6] (2016) | Non-NASH vs. NASH | Estimation, n=223 (non-NASH, n=176; NASH, n=47) | NASH ClinLipMet Score (glutamate, isoleucine, glycine, lysophosphatidylcholine 16:0, phosphoethanolamine 40:6, AST, fasting insulin, PNPLA3 genotype) | AUROC, 0.866; sensitivity, 86%; specificity, 72% |
| Validation, n=95 (non-NASH, n=71; NASH, n=24) | |||||
| Mayo et al. [7] (2018) | NAFL vs. NASH | Discovery, n=467 (control, n=90; NAFL, n=246; NASH, n=131) | 20 triglyceride species and BMI | AUROC, 0.95; sensitivity, 83%; specificity, 94% | |
| Validation, n=192 (control, n=7; NAFL, n=109; NASH, n=76) | |||||
| Barr et al. [8] (2012) | NAFL vs. NASH | Discovery (n=374) | BMI-dependent metabolic profile of 292 metabolites and 51 unidentified variables | AUROC, 0.84; sensitivity, 62%; specificity, 97% | |
| Validation (n=93) | |||||
| Bril et al. [9] (2018) | NAFL vs. NASH | 220 (no NAFLD, n=66; NAFL, n=39; NASH, n=115) | 20 triglyceride species and BMI | AUROC, 0.69 | |
| Caussy et al. [12] (2019) | F0ŌĆōF2 vs. F3ŌĆōF4 | Derivation, n=156 (F0ŌĆōF2, n=133; F3ŌĆōF4, n=23) | 8 lipids (5alpha-androstan-3beta monosulfate, pregnanediol-3-glucuronide, androsterone sulfate, epiandrosterone sulfate, palmitoleate, dehydroisoandrosterone sulfate, 5alphaandrostan-3beta disulfate, glycocholate), taurine, fucose | AUROC, 0.94; sensitivity, 90%; specificity, 79% | |
| Validation 1, n=142 (MRE <3.63 kPa, n=131; MRE Ōēź3.6 kPa, n=11) | |||||
| Validation 2 (n=59) | |||||
| Caussy et al. [13] (2020) | F0ŌĆōF2 vs. F3ŌĆōF4 | 427 (F0ŌĆō2, n=229; F3ŌĆōF4, n=197) | Baseline (11,12-DIHETE, tetranor 12-HETE, adrenic acid, and 14, 15-DIHETE), Ōēź1 stage improvement in fibrosis at 24-week follow-up (5-HETE, 7,17-DHDPA, adrenic acid, arachidonic acid, eicosapentaenoic acid, 16-HDOHE, and 9-HODE) | AUROC, 0.74 (follow-up) | |
| Perakakis et al. [14] (2019) | F0 vs. F1ŌĆōF4 | 80 (control, n=49; NAFL, n=15; NASH, n=16) | 10 lipids, 5 glycans, 5 fatty acids | AUROC, 1.0; sensitivity, 97%; specificity, 99% | |
| Ogawa et al. [15] (2020) | Hepatocellular ballooning | 132 (non-ballooning, n=83; ballooning, n=49) | Type IV collagen 7S, choline, LPE (e-18:2) | AUROC, 0.846; sensitivity, 89.4%; specificity, 71.4% | |
| Amino acids | |||||
| Gaggini et al. [20] (2018) | F0ŌĆōF2 vs. F3ŌĆōF4 | 64 (control, n=20; nonobese NAFLD, n=29; obese NAFLD, n=15) | Glutamate-serine-glycine index | OR, 122 (P=0.004) | |
| Grzych et al. [25] (2020) | NAFL vs. NASH vs. NASH cirrhosis | Discovery, n=213 (control, n=69; NAFL, n=78; NASH, n=23; NASH cirrhosis, n=15; non-NASH cirrhosis, n=28) | Glycocholic acid, taurocholic acid, phenylalanine, branched-chain amino-acids | Accuracy, 94.0%; sensitivity, 94.1%; specificity, 93.8% | |
| Validation, n=94 (control, n=44; NAFL, n=34; NASH, n=10; NASH-cirrhosis, n=6) | |||||
| Bile acid | |||||
| Legry et al. [29] (2017) | Advanced fibrosis, NASH, hepatocellular ballooning | 152 (control, n=50; NAFLD, n=102) | Keto-deoxycholic acid, 7-ketolithocholic acid | Keto-deoxycholic acid with advanced fibrosis (OR, 4.2), NASH (OR, 24.5) and hepatocellular ballooning (OR, 18.7); 7-ketolithocholic acid with NASH (OR, 9.4) and ballooning (OR, 5.9) | |
F0ŌĆōF4 represents the stage of hepatic fibrosis on a scale from 0 to 4.
NAFL, nonalcoholic fatty liver; NASH, nonalcoholic steatohepatitis; AST, aspartate aminotransferase; PNPLA3, patatin-like phospholipase domaincontaining-3; AUROC, area under the receiver operating characteristic; BMI, body mass index; NAFLD, nonalcoholic fatty liver disease; MRE, magnetic resonance elastography; DIHETE, dihydroxy-5Z,8Z,14Z,17Z-eicosatetraenoic acid; HETE, hydroxyeicosatetraenoic acid; DHDPA, dihydroxy-(8Z,10,13,15E,19Z)-docosapentaenoic acid; HDOHE, hydroxy-docosahexaenoic acid; HODE, hydroxy-octadecadienoic acid; LPE, lysophosphatidylethanolamine; OR, odds ratio.
Abbreviations
REFERENCES
- TOOLS
-
METRICS

- ORCID iDs
-
Hwi Young Kim

https://orcid.org/0000-0003-0723-1285 - Related articles
-
Recent updates on pharmacologic therapy in non-alcoholic fatty liver disease2024 January;30(1)
Letter regarding ŌĆ£Risk factors in nonalcoholic fatty liver diseaseŌĆØ2023 October;29(4)
Lean or Non-obese Nonalcoholic Fatty Liver Disease Patients: Are They Really Lean?2023 October;29(4)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



