Stereotactic body radiation therapy for small (≤5 cm) hepatocellular carcinoma not amenable to curative treatment: Results of a single-arm, phase II clinical trial
Article information
Abstract
Background/Aims
Stereotactic body radiation therapy (SBRT) is used as an alternative ablative treatment in patients with hepatocellular carcinoma (HCC) not suitable for curative treatments. The purpose of this prospective study was to evaluate the long-term efficacy of SBRT for small (≤5 cm) HCCs.
Methods
A phase II, single-arm clinical trial on SBRT for small HCCs was conducted at an academic tertiary care center. The planned SBRT dose was 45 Gy with a fraction size of 15-Gy over 3 consecutive days. The primary endpoint was 2-year local control rate. Radiologic responses were assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1) and the modified RECIST criteria.
Results
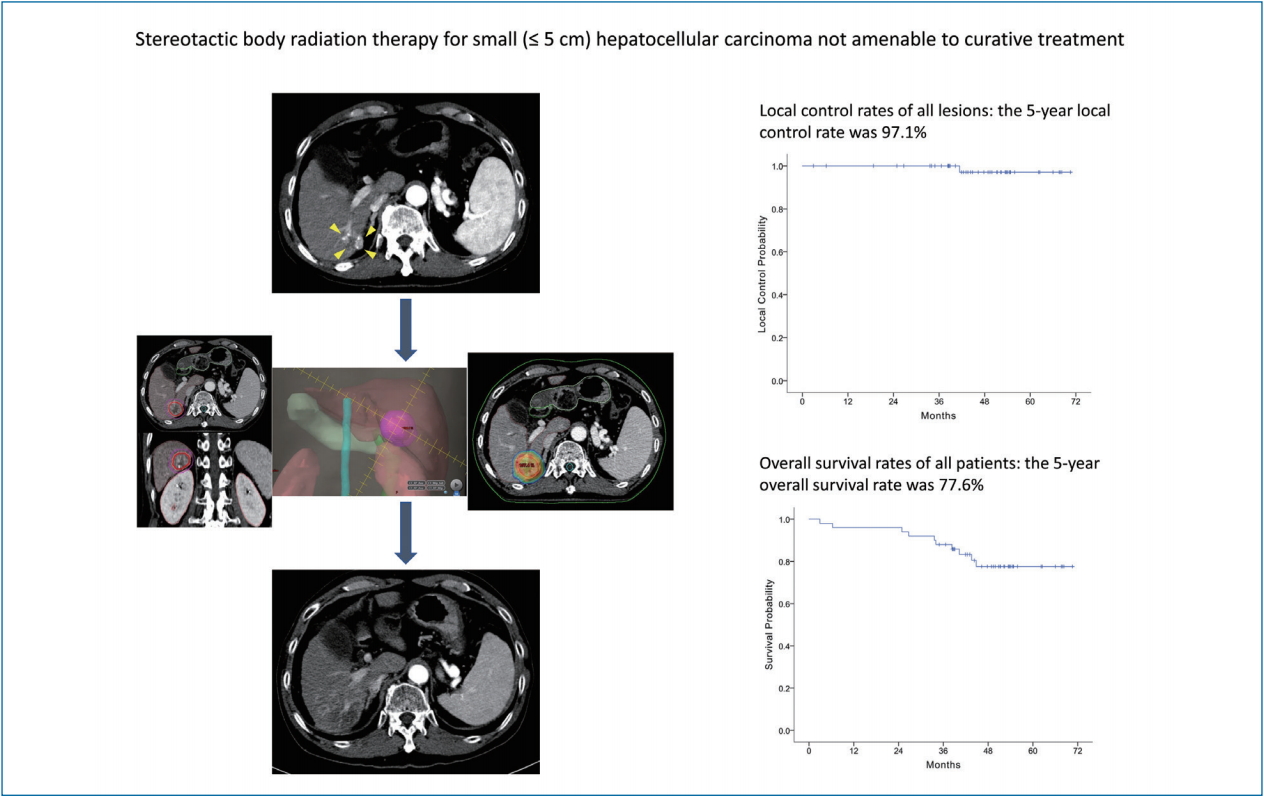
Between 2013 and 2016, 50 patients (53 lesions) were enrolled, with a median follow-up period of 47.8 months (range, 2.9–70.6). Patients’ age ranged from 41 to 74 years, and 80% were male. Median tumor size was 1.3 cm (range, 0.7–3.1). The 2- and 5-year local control rates were 100% and 97.1%, respectively. The 5-year overall survival rate was 77.6%. Six months after SBRT, radiologic responses were evident in 44 lesions (83%) according to the RECIST criteria and 49 (92.4%) according to the modified RECIST criteria. None of the patients showed grade ≥3 adverse events.
Conclusions
SBRT showed excellent results as an ablative treatment for patients with small HCCs while showing minimal toxicities. SBRT can be a good alternative for both curative and salvage intents in patients with HCCs that are unsuitable for curative treatments.
Graphical Abstract
INTRODUCTION
Liver cancer is the second leading cause of cancer-related mortality, and hepatocellular carcinoma (HCC) accounts for approximately 90% of primary liver cancers at diagnosis [1,2]. Moreover, the incidence of liver cancer worldwide is predicted to increase until 2030, although viral hepatitis-related liver cancer rates are expected to decrease [3]. According to the current treatment guidelines for HCC, hepatic resection, liver transplantation, and radiofrequency ablation (RFA) are used as curative treatments for very early and early stage HCCs [1,4,5]; however, these curative treatments cannot be applied in all patients with early stage HCCs considering the baseline liver function, availability of donor organs, and tumor location. Therefore, stereotactic body radiation therapy (SBRT) has been used as an alternative, non-invasive local ablative treatment in patients with very early or early stage HCCs for which these curative treatments cannot be applied [6-10]. Owing to recent advances in radiotherapy techniques and radiosensitive characteristics of HCC, SBRT has shown excellent local tumor control rates as well as minimal treatment-related toxicity, especially for small (≤5 cm) HCCs that are not suitable for curative treatments [8,9,11-13]. As a result of these promising outcomes, SBRT was regarded as an alternative treatment to thermal ablation for patients with Barcelona Clinic Liver Cancer stage A according to the recently updated practice guidance by the American Association for the Study of Liver Disease (AASLD) [5].
Several prospective clinical trials have been conducted to assess the efficacy of SBRT in patients with HCC; however, the eligibility criteria of those studies varied, especially regarding the stage of HCC and tumor size [6,7,9,14,15]. Considering the powerful ablative effect of SBRT in patients with HCC, a more focused prospective study is needed to support the clinical benefits of SBRT. Thus, we conducted a phase II clinical trial to assess the clinical outcomes of SBRT in patients with small (≤5 cm) HCCs that are not suitable for curative treatments.
MATERIALS AND METHODS
Study outline and participants
This was a single-arm, phase II clinical trial conducted at Asan Medical Center, Seoul, Republic of Korea, and registered at the Clinical Research Information Service (registration number: KCT0000625). Patients aged 20 years or older with a diagnosis of HCC were eligible. HCC was diagnosed by pathologic confirmation and/or typical findings on 4-phase dynamic computed tomography (CT) or magnetic resonance images (MRI) using a hepatocyte-specific contrast agent according to the AASLD criteria [16]. The study protocol was approved by the Institutional Review Board of Asan Medical Center (2012-0768), and written informed consent was obtained from all patients prior to enrollment.
The inclusion criteria were as follows: primary or recurrent HCC with a Child-Pugh class A hepatic function; an Eastern Cooperative Oncology Group performance status score of 0 or 1; HCCs with longest diameter of ≤5 cm, and ≤3 lesions with a sum of diameter ≤5 cm; not suitable for surgery because of liver cirrhosis, insufficient remnant liver for resection, or patient refusal to undergo surgery; not suitable for RFA due to the size (>3 cm in the longest diameter) or location (e.g., liver surface, near to the bile duct or large vessels, near the dome or pericardium) of HCC, undetectable HCC on ultrasonography, or bleeding tendency of patients; lesion non-visibility on hepatic angiogram for transarterial chemoembolization (TACE) or an incomplete response after TACE according to the modified Response Evaluation Criteria in Solid Tumors (RECIST); a sufficient distance (>2 cm) between HCC and radiosensitive organs such as the stomach, duodenum, esophagus, and large bowel; and evidence of an adequate residual functional liver volume (>700 mL).
Patients were excluded if they had macroscopic vascular invasions, extrahepatic metastasis, uncontrolled ascites, hepatic encephalopathy, transaminase level >200 IU/L, history of liver transplantation or radiotherapy, an active gastric or duodenal ulcer, other uncontrolled comorbidities, or malignant tumors.
Intervention
The simulation and target volume delineation for SBRT were identical to those used in our previous studies [10,17,18]. All patients were immobilized with a vacuum cushion in the supine position. Four-dimensional (4D) CT scanning was performed using a 16-slice CT system (GE LightSpeed RT 16; GE Healthcare, Waukesha, WI, USA). The 4D-CT images, synchronized with the respiratory data, were sorted into 10 CT series according to the respiratory phase (Advantage 4D version 4.2; GE Healthcare). Patients’ respiratory data were analyzed using a Real-time Position Management gating system (Varian Medical Systems, Palo Alto, CA, USA). Three gold seeds (CIVICO Medical Solutions, Kalona, IA, USA) were implanted in the hepatic parenchyma around the tumor prior to CT simulation. The gold seeds were not implanted in patients who had surgical clips or compact iodized oil around the viable HCC after prior treatments, or those with uncontrollable bleeding tendencies [19].
The gross tumor volume (GTV), as determined by dynamic enhanced CT or MRI, included an enhanced HCC at the end-expiratory phase of the 4D-CT image. The clinical target volume was equal to the GTV. The internal target volume (ITV) was determined as the sum of the individual GTVs as defined within the gated phases of respiration. The planning target volume (PTV) was added with a 0.5-cm margin from the ITV [10].
SBRT planning was performed using a 3-dimensional radiotherapy planning system (Eclipse; Varian Medical Systems) that used the two arcs of volumetric-modulated arc therapy technique using a 10-MV flattening filter-free beam using a linear accelerator (TrueBeam STx; Varian Medical Systems) [18]. A total dose of 45 Gy was prescribed using 15 Gy per fraction over 3 consecutive days. The total dose was determined based on our prescription guidelines including the following: 1) the maximum dose allowed to 700 mL of normal liver was estimated to be 15 Gy in three fractions and 2) the mean dose to normal liver did not exceed 13 Gy in three fractions. The dose constraints to adjacent critical normal organs were as follows: 1) 2 mL of the esophagus or large bowel were limited to a total dose of <21 Gy; 2) 2 mL of the stomach or duodenum were limited to <18 Gy; and 3) 2 mL of the spinal cord were limited to 18 Gy [10]. The beam delivery was performed with an image-guidance and a respiratory-gated beam delivery technique using an On-Board Imager (Varian Medical Systems).
Outcomes and evaluation
The primary endpoint of this study was 2-year local control rate. Local control was defined as no evidence of tumor progression of the treated lesion. Local tumor progression was defined as recurrence within the PTV, intrahepatic recurrence was defined as recurrence within the liver outside the PTV, and extrahepatic metastasis was defined as recurrent disease at any site other than the liver. Secondary endpoints were radiologic response rates, recurrence-free survival, and overall patient survival.
Tumor measurements and response evaluation were conducted by a radiologist (SYK) with more than 10 years of experience in HCC imaging. Radiologic response was defined as the combined number of complete response and partial response and evaluated by liver dynamic CT at 2-, 4-, and 6-months after SBRT completion according to both the RECIST version 1.1 and the modified RECIST criteria [20,21]. After 6 months, regular follow-up examinations were performed at 3-month intervals. After completion of the 2-year study period, patients continued to receive treatment and were followed-up for disease status and survival until March 31, 2019. Laboratory tests were performed at every visit, including blood counts, chemical profiles, prothrombin time, alpha-fetoprotein, and protein induced by vitamin K absence or antagonistsII levels. Adverse events were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCICTCAE; version 4.03). Radiation-induced liver disease was also graded according to CTCAE or any decline in liver function using the worsening of Child-Pugh score ≥2 in the absence of progressive disease within 3 months after SBRT.
Statistical analysis
The primary data set for efficacy analyses comprised all enrolled patients (intention-to-treat analysis). For sample size calculation, we assumed that the 2-year local control rate would be >90%, in contrast to <75% reported in previous studies on SBRT [15,22]. Considering a 10% of drop-out rate, according to the Simpson’s phase 2 single-stage design, a total of 50 patients was required in this study for a two-sided test with 5% significance level and 90% power to detect a difference in local control rate of 15%.
Overall and recurrence-free survival rates were estimated from the SBRT start date to the date of death, the last follow-up examination, or to the date of tumor recurrence. The probability of cumulative survival was calculated using the Kaplan-Meier method. Univariate and multivariate analyses were performed to evaluate the association of covariates with overall and recurrence-free survivals. Cox proportional hazards model with backward elimination was used to identify risk factors in the multivariate model. All statistical analyses were performed using the SPSS software (version 21; IBM SPSS Statistics, Armonk, NY, USA).
RESULTS
Study population
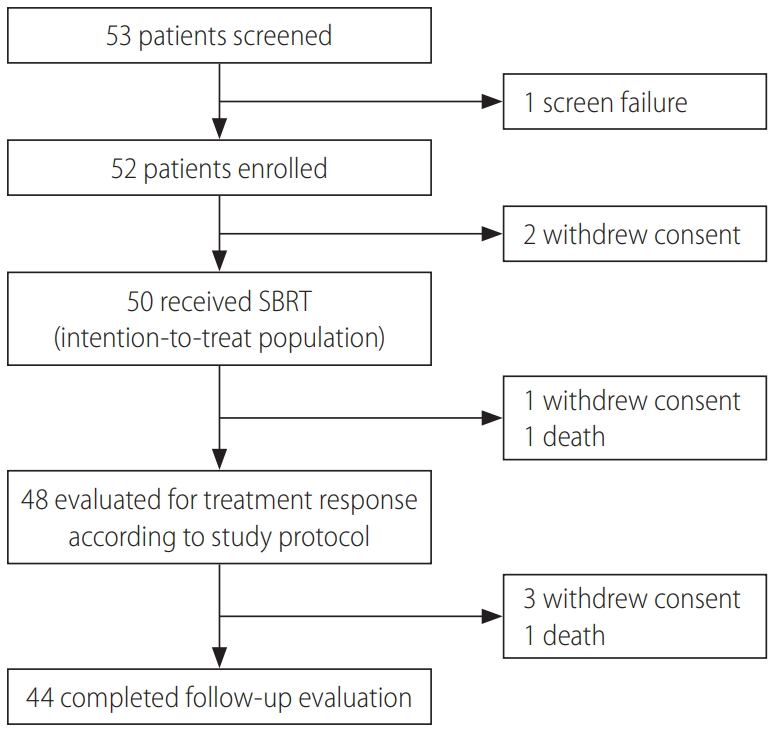
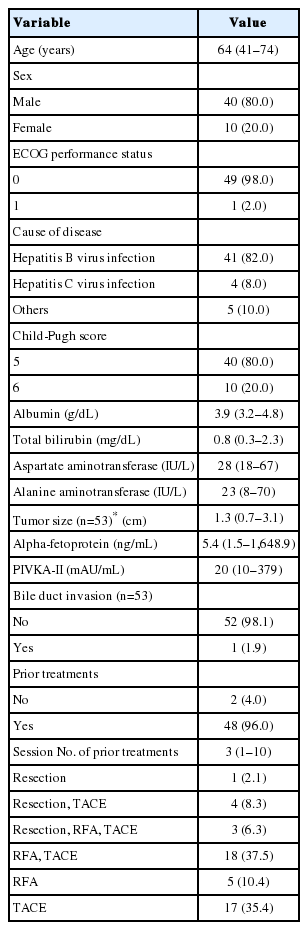
Between April 4, 2013 and August 19, 2016, 50 patients were included in the intention-to-treat analysis (Fig. 1). Given that each of three patients had two viable HCCs that were simultaneously treated with SBRT, a total of 53 lesions were included in this study. Table 1 summarizes the patient characteristics. The study population was mostly males (80%), with a median age of 64 years (range, 41–74). Chronic hepatitis B virus (HBV) infection was the main cause of background liver disease. Median tumor size was 1.3 cm (range, 0.7–3.1) using either the RECIST or the modified RECIST measurements, and one patient had HCC with a segmental bile duct invasion prior to SBRT. The locations of the HCC lesions were as follows: subcapsular area (26, 49.1%), perivascular area (15, 28.3%), near the dome (10, 18.8%), and previous RFA margin (2, 3.8%). All patients received a planned dose of 45 Gy in three fractions (prescription, 91–100% of isodose line) for target lesions. Only two patients (4%) were treatment-naïve, and all other patients had received various courses of locoregional therapies before receiving SBRT (median, 3 courses). However, additional locoregional treatments were not performed on recurrent or residual viable HCCs if SBRT was considered according to the study protocol.
Local control rates and radiologic response
At the completion of the 2-year study period, 44 patients (47 lesions) completed the follow-up evaluations according to the study protocol (Fig. 1). During the follow-up period, local recurrence was observed in one (1.8%) out of the 53 lesions, resulting in 2- and 5-year local control rates of 100% and 97.1%, respectively (Fig. 2A; estimated local recurrence-free survival in Supplementary Fig. 1).
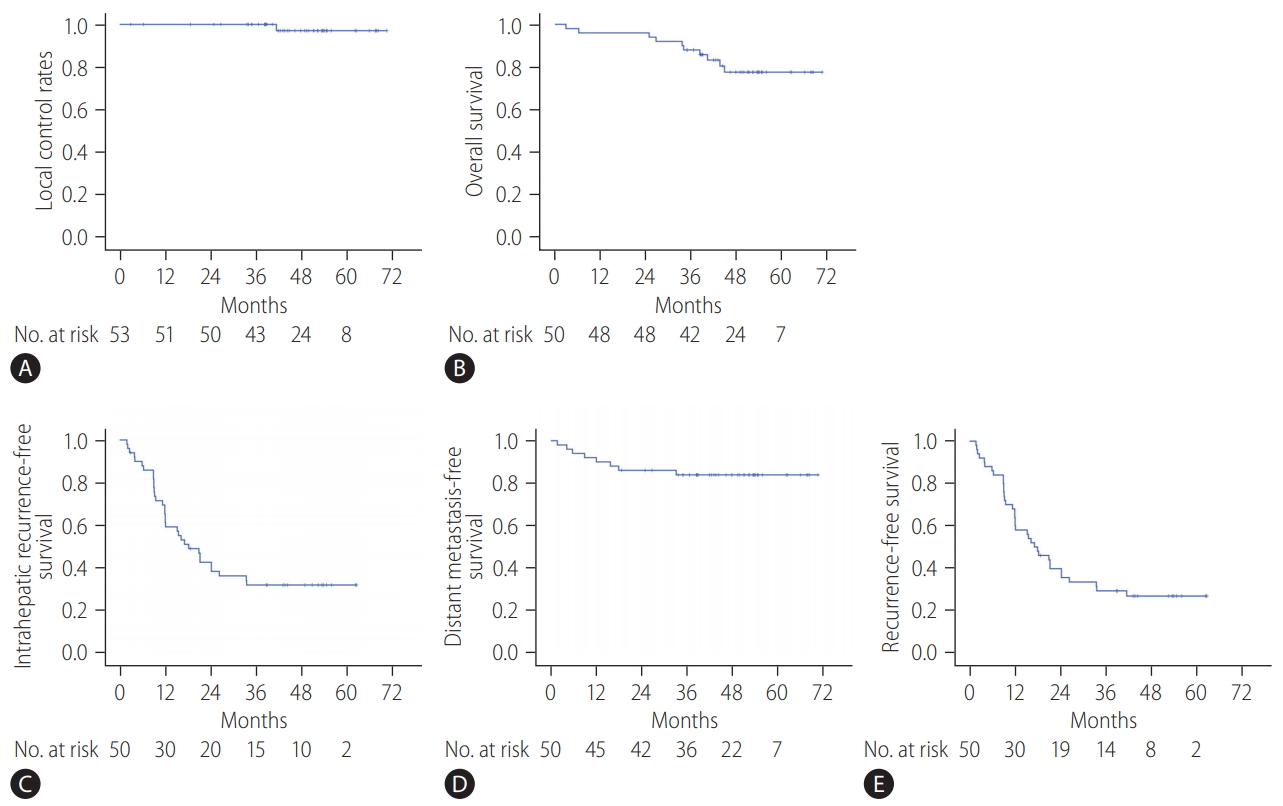
(A) Local control rates of all lesions after stereotactic body radiation therapy, (B) overall survival rates of all patients, (C) intrahepatic recurrence-free survival rates of all patients, (D) distant metastasis-free survival rates of all patients, and (E) recurrence-free survival rates of all patients.
At 2 months after completion of SBRT, all lesions underwent response evaluation; radiologic responses were observed in 19 (35.8%) and 24 (45.3%) lesions according to the RECIST and the modified RECIST criteria, respectively. At 4 months, 37 (69.8%) and 43 (81.1%) lesions showed responses according to the RECIST and the modified RECIST criteria, respectively. At the final response evaluation of 6 months after completion of SBRT, 44 (83.0%) and 49 (92.4%) lesions had achieved radiologic responses according to the RECIST and the modified RECIST criteria, respectively (Table 2). A representative case is shown in Supplementary Figure 2.
Overall and recurrence-free survival rates
The median follow-up period of participants was 47.8 months (range, 2.9–70.6). By the last follow-up, 40 patients were alive. The 2- and 5-year overall survival rates were 96.0% and 77.6%, respectively (Fig. 2B). Thirty-six patients were diagnosed as having tumor recurrences on follow-up imaging studies (CT, 26 patients; both CT and MRI, 10 patients). Among them, intrahepatic (i.e., outside the PTV) HCC recurrence was the main cause of failure (33 of 50 patients), and distant metastasis also developed in eight patients during follow-up. The rates of 5-year intrahepatic recurrence-free survival, distant metastasis-free survival, and recurrence-free survival were 32.0%, 83.9%, and 26.8%, respectively (Fig. 2C-E). Multivariate analysis revealed that tumor size was significantly associated with overall survival (hazard ratio [HR], 2.504; 95% confidence interval [CI], 1.031–6.084; P=0.043) and recurrence-free survival rates (HR, 2.791; 95% CI, 1.604–4.858; P<0.001) (Supplementary Tables 1, 2).
Treatment-related toxicity
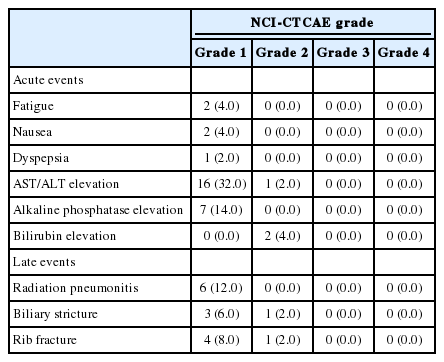
SBRT-related toxicities are summarized in Table 3. All patients received the planned SBRT without any interruptions due to intolerable side effects. Fatigue and anorexia were the most common acute toxicities, which were mostly mild (NCI-CTCAE grade 1) (Table 3).
Two patients (4%) experienced elevations in the Child-Pugh score to ≥2. Of these, one patient had a Child-Pugh score 8 hepatic function and also showed peritoneal seeding nodules at 2 months after completion of SBRT; this patient died due to hepatic failure at 2.9 months after SBRT. Serious adverse events were reported in seven patients, including compression fracture in lumbar spine, variceal bleeding, cholangitis, fungal sinusitis, dyspnea, abdominal pain, and gallbladder stone (all n=1), all of which were regarded as unrelated to SBRT.
There were no gastrointestinal complications such as bleeding or perforation during follow-up. Five patients (10%) developed rib fractures, which did not require any specific treatment and improved spontaneously. Four patients (8%) developed grade 1 or 2 biliary strictures, and six patients (12%) experienced grade 1 radiation pneumonitis in the right lower lung fields (Table 3).
DISCUSSION
In this phase II clinical trial of patients with small (≤5 cm) HCCs, SBRT was associated with high rates of local tumor control (100% at 2 years and 97.1% at 5 years), radiologic response (92.4% at 6 months by the modified RECIST criteria), and overall survival (77.6% at 5 years) while showing minimal treatment-related toxicity. One patient experienced hepatic failure 2 months after SBRT, the cause of which was unclear. These results show clinical benefits of using SBRT as an ablative treatment for small HCCs that are not amenable to hepatic resection, RFA, or even TACE.
Multiple prospective and retrospective studies have been conducted to evaluate the efficacy of SBRT for HCC, with many showing promising outcomes. However, the indications for SBRT varied, with some trials including patients with portal vein tumor thrombus [6,7,15] and others including patients with extrahepatic metastasis [15]. Moreover, a wide range of tumor sizes, from 1.0 cm to 23.1 cm, was included [6,7,9,14,15]. Thus, it was difficult to draw a consistent conclusion on the use of SBRT for HCC. As SBRT can be used for various clinical purposes from curative to palliative aims at the physician’s discretion, the results of curative intent SBRT on patients with early stage HCCs should be analyzed if the ablative effect of SBRT is the primary endpoint. Therefore, we designed the current trial by including patients with liver-confined, small (≤5 cm) HCCs without macroscopic vascular invasion. Similarly, Takeda et al. [9] conducted a phase II study on SBRT for early stage or small (≤4 cm) HCCs and reported favorable outcomes in terms of 3-year local control (96.3%) and 3-year liver-related cause-specific survival (72.5%). Other retrospective studies using ≤5–6 cm of maximum HCC tumor diameter as inclusion criteria also showed high local control rates [8,10-13,18].
In this study, 2- and 5-year local control rates were as high as 100% and 97.1%, respectively. Such high local control rates could be explained as follows. First, participants of this study had relatively smaller HCCs (range, 0.7–3.1 cm) than those of previous studies. With the exception of two patients, 48 (51 lesions) were enrolled with recurrent HCC after prior locoregional treatments. Hence, we decided to perform salvage therapies when typical recurrence was observed on regular follow-up images even if the lesion was small. As tumor size is one of the most important factors for local tumor control after SBRT [8,10,23-25], this may have led to the high local control rate in this study. Second, the established SBRT procedures and techniques at our institution, including 4D-CT, tumor localization on planning CT, respiratory-gated delivery, and a thorough image-guidance on each fraction, may have contributed to the improved clinical outcomes. We recently evaluated the targeting accuracy of our image-guided SBRT for HCC using post-radiotherapy MRI with hepatocyte-specific contrast agent and found that the set-up errors were less than 5 mm in cranio-caudal, right-left, and anterior-posterior directions with or without internal fiducial markers [19]. This high local tumor control could not be observed in early phase follow-up images. The timing of treatment response was evaluated by an experienced abdominal radiologist at regular intervals (2-, 4-, 6-month after SBRT) according to the study protocol. Response rates were less than 50% at 2 months; however, at 6 months after completion of SBRT, they were around 90% according to both the RECIST and modified RECIST criteria. This delayed response trend after SBRT is a potentially valuable finding that may aid decision-making processes for the management of HCC.
Although most of the enrolled patients had recurrent HCCs with a history of locoregional treatments, the overall survival rate in this study was as high as 77.6% at 5 years. The most likely reason for this promising result was that all patients had preserved liver functions according to the eligibility criteria. The main failure pattern was intrahepatic recurrence, with a cumulative intrahepatic recurrence of about 70%, which is similar to that of previous reports. Given that multicentric carcinogenesis in the context of chronic liver disease is considered as an important cause of intrahepatic recurrence, similar outcomes were observed after SBRT for solitary HCC or after RFA [9,26,27]. Therefore, a regular follow-up and an active decision of salvage therapies are necessary to control recurrent HCCs after SBRT.
Based on the encouraging outcomes of SBRT for HCC, some studies have compared the outcomes of locoregional therapies (e.g., hepatectomy, RFA, and TACE) with those of SBRT [28-31]. Of these, Hara et al. compared these two modalities in patients with small (≤3 cm) HCCs who received curative intent treatment and reported that the 3-year local recurrence rates in the SBRT and the RFA groups were 5.3% and 12.9% (P<0.01), respectively; these differences were more notable if the HCCs were attached to vessels (≤1 mm) (5.2% in the SBRT group, 25.5% in the RFA group; P<0.01) [28]. After propensity score matching, overall survival rates, cancer-specific mortality rates, and liver failure mortality rates were not significantly different between the two groups; and the authors concluded that SBRT was an acceptable alternative option for patients who were not candidates for RFA [28]. Although these studies had retrospective designs, their results warrant the need for well-designed clinical trials to delineate the clinical benefits of using SBRT as an ablative treatment for patients with HCC, especially for those with early stage diseases.
This study has the following limitations. First, the number of enrolled patients was relatively small, and the number of events (local recurrence: 1, death: 10) that occurred during follow-up was not enough to perform statistical analysis. Therefore, it was difficult to find novel results on the use of SBRT for HCC, compared with the previous reports. Second, HBV infection was the main cause (82%) of background liver disease; thus, the results of this study should be further confirmed in patients with HCCs unassociated with HBV. Finally, most of the enrolled patients had recurrent HCCs with a history of prior locoregional treatments; thus, further studies on SBRT in treatment-naïve patients with small HCCs are needed to confirm the role of SBRT as a curative treatment option. Nevertheless, focusing on the ablative effect of SBRT in patients with liver-confined, small (≤5 cm) HCCs, presenting the trends of radiologic response rates after SBRT measured at regular intervals, and long-term follow-up data could be considered valuable to report our prospective trial.
In conclusion, this phase II clinical trial demonstrated that SBRT was well-tolerated and resulted in high local control rates and promising overall survival rates in patients with small (≤5 cm) HCCs. Considering that tumors included in this study could not be treated by curative treatment modalities, SBRT can be considered a good alternative treatment option when hepatic resection or RFA cannot be applied.
Notes
Authors’ contribution
Sang Min Yoon: Conceptualization and design, investigation, acquisition of data, formal analysis, methodology, writing-original draft, and writing-review and editing. So Yeon Kim: Investigation, acquisition of data, formal analysis, methodology, and writing-review and editing. Young-Suk Lim: Investigation, formal analysis, methodology, and writing-review and editing. Kang Mo Kim: Investigation, methodology, and writing-review and editing. Ju Hyun Shim: Investigation, methodology, and writing-review and editing. Danbi Lee: Investigation, methodology, and writing-review and editing. Jihyun An: Investigation, methodology, and writing-review and editing. Jinhong Jung: Investigation, methodology, and writing-review and editing. Jong Hoon Kim: Investigation, methodology, and writing-review and editing. Han Chu Lee: Conceptualization and design, funding acquisition, investigation, acquisition of data, formal analysis, methodology, supervision, writing-original draft, and writing-review and editing. All authors approved the final version of manuscript.
Conflicts of Interest: The authors have no conflicts to disclose.
Acknowledgements
We thank Won Hee Jeong, RN and Jina Park, RN for their operational oversight of this clinical trial. We also thank Dr. Joon Seo Lim from the Scientific Publications Team at Asan Medical Center for his editorial assistance in preparing this manuscript.
This study was supported by a grant (#2012-0768) from the Asan Institute for Life Sciences of Asan Medical Center, Seoul, Republic of Korea.
SUPPLEMENTAL MATERIAL
Supplementary material is available at Clinical and Molecular Hepatology website (http://www.e-cmh.org).
Estimated local recurrence-free survival rate.
A representative case of a 74-year-old woman who received stereotactic body radiation therapy (SBRT) for recurrent hepatocellular carcinoma (HCC). (A) Arterial phase computed tomography (CT) scan at screening, showing a 1.5-cm viable HCC (yellow arrowheads) in segment VII. (B) The tumor (yellow arrowheads) showed washout on delayed phase CT scan (C) SBRT was performed with a total dose of 45 Gy in three fractions prescribed in the 97% isodose line. (D) At 2 months after SBRT, the patient showed complete radiologic response according to the modified RECIST criteria. (E) CT scan at 4 months after SBRT. (F) CT scan at 6 months after SBRT. (G) CT scan at 12 months after SBRT. (H) CT scan at 24 months after SBRT. (I) By the last follow-up at 40 months, no recurrence was observed on the liver dynamic CT scan. Radiation-induced parenchymal change in the right lower lung field was observed at 4 months after SBRT (E) and its serial changes during follow-up (F-I: yellow arrow).
Factors related to recurrence-free survival after stereotactic body radiation therapy
Factors related to overall survival after stereotactic body radiation therapy
Abbreviations
4D
four-dimensional
AASLD
American Association for the Study of Liver Diseases
CI
confidence interval
CT
computed tomography
GTV
gross tumor volume
HBV
hepatitis B virus
HCC
hepatocellular carcinoma; HR, hazard ratio
ITV
internal target volume
MRI
magnetic resonance image
NCI-CTCAE
National Cancer Institute Common Terminology Criteria for Adverse Events
PTV
planning target volume
RECIST
Response Evaluation Criteria in Solid Tumors
RFA
radiofrequency ablation
SBRT
stereotactic body radiation therapy
TACE
transarterial chemoembolization
References
Article information Continued
Notes
Study Highlights
This phase II clinical trial on SBRT for small (≤5 cm) HCCs resulted in a 5-year local control rate of 97.1% and a 5-year overall survival rate of 77.6% while showing minimal treatment-related toxicity. Radiologic response was observed in about 90% of treated lesions at 6 months after SBRT completion. Considering that none of the tumors included in this study could be treated by surgery or percutaneous ablative therapies, SBRT can be a viable alternative for both curative and salvage intents in patients with HCCs that are unsuitable for curative treatments.