The dilemma of differentiating between acute hepatitis B and chronic hepatitis B with acute exacerbation: Is quantitative serology the answer?
Article information
Abstract
Background/Aims
Acute exacerbations of chronic hepatitis B (CHB-AEs) are common in endemic areas and are often presumed to be acute hepatitis B (AHB) due to their similarities in clinical and serological pictures, presenting a major diagnostic dilemma. This study aimed to identify laboratory markers for differentiating between the two groups, and to establish the cut-off value for significant markers.
Methods
A retrospective analysis of records was conducted for patients who presented with clinical features of acute hepatitis along with hepatitis B surface antigen (HBsAg) and IgM antibody to hepatitis B core antigen (IgM anti-HBc) positivity from May 2015 to May 2017. A total of 172 patients were enrolled and grouped as AHB (n=89) and CHB-AE (n=83) based on their history of hepatitis B virus infection and duration of HBsAg persistence. Virological and biochemical parameters were analyzed and compared. Cut-off values, sensitivity, and specificity of the variables were calculated.
Results
The median value of signal by cut-off (S/Co) ratio for IgM anti-HBc was significantly higher in AHB group (30.44) compared to CHB-AE group (8.63) with a sensitivity and specificity of 97% and 84%, respectively, at a cut-off of 20.5 (P<0.01). The mean international normalized ratio (INR) was significantly greater in CHB-AE (1.88±1.24) group compared to AHB group (1.62±0.17) with a sensitivity and specificity of 57.9% and 45.1%, respectively, at a cut-off value of 1.27.
Conclusions
A value of 20.5 S/Co of IgM anti-HBc and 1.27 INR could be helpful in differentiating between AHB and CHB-AE. (Clin Mol Hepatol 2020;26:187-195)
Graphical Abstract
INTRODUCTION
Hepatitis B virus (HBV) infection is a substantial global health problem, with more than 2 billion people infected worldwide and 257 million cases being chronic hepatitis [1,2]. In India, it is estimated that about 40 million people are chronically infected with the virus, with a prevalence of 3.7% [3]. HBV infection results in a spectrum of disease entities ranging from asymptomatic carrier state to the most severe form of chronic active hepatitis B [4].
A large percentage of patients with chronic active hepatitis may frequently show a changing pattern, including acute exacerbations in liver injury along with episodes of normal liver function. This reflects the dynamic interplay between the immune response of human body and viral replication [5]. According to the 2015 Asian Pacific Association for the Study of the Liver (APASL) clinical practice guidelines on the management of hepatitis B, acute exacerbations in chronic hepatitis B (CHB-AEs) are usually defined as intermittent elevations of aminotransferase to more than five times the upper limit of normal and more than twice the baseline value [6]. In HBV endemic areas, CHB-AEs are common and may often be the first sign of the disease. Over 50% of such patients are presumed to have acute hepatitis B (AHB) due to similar clinical and serological pictures, thereby posing a major diagnostic dilemma [7-10].
The need to differentiate between CHB-AE and AHB is worthy of our attention due to their different prognosis which requires different types of therapeutic intervention. Most of the patients with AHB show complete clinical improvement and resolve spontaneously. Only a small number of those with severe/fulminant disease may require treatment while those with CHB-AE usually require therapy for hepatic decompensation, and high mortality may occur as a result of hepatocellular dysfunction [11]. Here, a simple and reliable serological marker would come in handy to differentiate between these two conditions. The amount of available data on the use of serological assays in diagnosing CHB-AE is very limited, and there are variations in the inclusion and differentiation criteria of different studies. Immunoglobulin M antibody to the hepatitis B core antigen (IgM anti-HBc) has been considered an important diagnostic marker for AHB [12,13]. However, since about 20% to 27.5% of CHB-AE patients have IgM anti-HBc positivity with the fully automated, quantitative analysis method, these patients could clinically present as AHB [10]. Therefore, a simple, effective, and reliable serological marker with a cut-off value is required to differentiate between these two conditions. Very few studies on this topic have been conducted in India. In this study, we aimed to identify the difference in serological markers of AHB and CHB-AE, and to figure out an optimal cut-off value for the serological monitoring of HBV.
MATERIALS AND METHODS
This study was performed at a tertiary care liver center in India. After the approval from the Institutional Ethics Committee (IEC/2018/58/MA03) and review board, a retrospective analysis of records was done on all patients who presented with acute viral hepatitis with hepatitis B surface antigen (HBsAg) and IgM anti-HBc positivity between May 2015 and May 2017 [6]. Detailed clinical information regarding the onset of illness, presenting signs and symptoms, and previous history of such episodes of HBV infection were obtained from the Hospital Information System. Patients coinfected with hepatitis A virus, hepatitis C virus, hepatitis E virus, and human immunodeficiency virus were excluded from the study. After careful review of the subjects’ history as well as their clinical and serological profiles, 172 patients were included and divided into two groups: AHB (89, 51.7%) and CHB-AE (83, 48.3%). AHB group included patients with clinical signs and symptoms of AHB without a history of past HBV infection, as well as HBsAg not persisting for more than 6 months. CHB-AE group included HBV carriers with HBsAg antigen persisting for more than 6 months, who had more than five times the upper limit of normal and more than twice the baseline value of aminotransferase. Baseline characteristics such as age, sex, and biochemical parameters including serum levels of total bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), AST/ALT ratio, international normalized ratio (INR), AST platelet ratio index (APRI) score, and albumin, were noted. Out of 83 patients from CHB-AE group, 60 (72.2%) were cirrhotic and 23 (27.7%) were non-cirrhotic using 1.5 as the higher cut-off of APRI score. Biochemical and virological profiles of the two groups were compared to establish the diagnostic markers for their differentiation.
Various serological markers, such as IgM anti-HBc, quantitative HBsAg (qHBsAg), hepatitis B e antigen (HBeAg), and antibody to HBeAg (anti-HBe), were tested on commercial Chemiluminescence Immunoassay (CLIA)-based platform (Architect i1000SR; Abbott Diagnostics, Abbott Park, IL, USA). Chemiluminescence immunoassay on the Abbott Architect is a two-step immunoassay with flexible assay protocols called chemiflex, which utilizes paramagnetic particles coated with the respective antigen/antibodies to which human serum is incubated. After washing, acridine-labelled conjugate was added followed by pre-trigger and trigger solutions, and the resulting chemilluminiscent reaction was measured as relative light units (RLUs). A direct relationship was shown between the amount of analyte in the sample and the RLUs detected by Architect System optics. Results were calculated as normalized signal cut-off (S/Co) ratios obtained by measuring the signal strength of sample and the signal strength of an internal cut-off. IgM anti-HBc positivity was defined by an S/Co ratio ≥1.0. The concentration of qHBsAg was determined using a previously generated Architect HBsAg calibration curve, which allows the quantitation of HBsAg from 0.05 to 250 IU/mL. Further dilution of samples was done to quantitate higher values. S/Co ≥1 was considered reactive for HBeAg, while S/Co ≤1 was considered as reactive for anti-HBe. Real time polymerase chain reaction was done for HBV DNA quantitation using COBAS TaqMan HBV test (Roche Diagnostics; GmbH, Mannheim, Germany), which has a lower limit of detection of 6 IU/mL and linear range of 29 to 1.1×10 8 IU/mL.
Statistical analysis
Quantitative variables were expressed as mean±standard deviation, or as median with range. Qualitative variables were expressed as numbers with percentage. HBV DNA levels and qHBsAg values were logarithmically transformed for analysis. Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) software for Windows version 22.0 (IBM SPSS, Armonk, NY, USA). Categorical data were compared using chi square test or Fischer exact test, as appropriate. Continuous data between the two groups were compared using Student’s t test or Mann-Whitney test, as appropriate. P value <0.05 was considered statistically significant. To identify the independent factors that were significantly associated with AHB, multiple logistic regression analysis was conducted using forward stepwise likelihood ratio. Selected variables with P value <0.05 on univariate analysis were included in regression analysis. To determine the optimal cut-off value of the variables for differentiating AHB from CHB-AE, the receiver operating characteristic (ROC) curves were plotted. The areas under ROC (AUROC) curves of identified factors were calculated. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were also calculated.
RESULTS
Baseline characteristics of the study population are described in Table 1. The enrolled population was divided into two groups: AHB (89, 51.7%) and CHB-AE (83, 48.3%). Both of the groups were comparable in all parameters except for AST and ALT being significantly higher in AHB group, reflecting the intensity of necroinflammation, and INR was significantly greater in CHB-AE group.
Comparison of virological parameters
Various viral markers were compared between the two groups (Table 2). To decrease the variability of data and make data conform more closely to the normal distribution, HBV DNA values were log-transformed as log10 IU/mL. No significant difference was found between AHB and CHB-AE patients in terms of median values of viral markers, based on semiquantitative analysis of test results. However, S/Co values between the two groups differed significantly in median levels of IgM anti-HBc (30.4 vs. 8.6, P <0.001).
Finding independent predictors for CHB-AE in comparison to AHB
Multiple logistic regression analysis of various factors revealed higher IgM anti-HBc titers and low INR as independent predictors of AHB as compared to CHB-AE (Table 3). Greater than 80% of correct predicting capability was seen in the combination of these two markers.
Diagnostic value of IgM anti-HBc and INR as independent predictors alone and in combination for the differentiation between AHB and CHB-AE
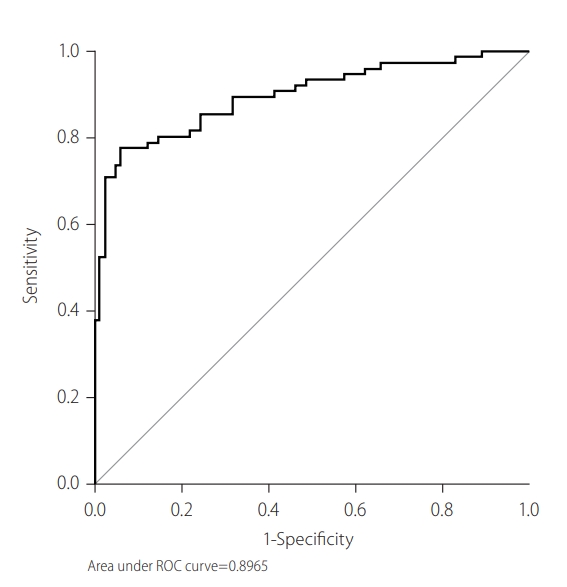
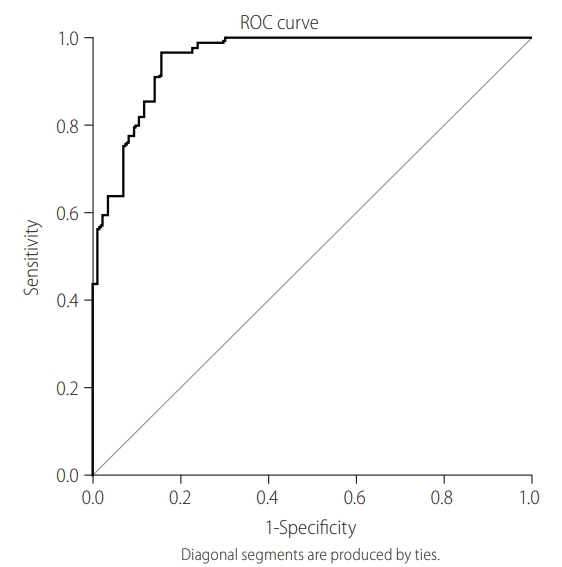
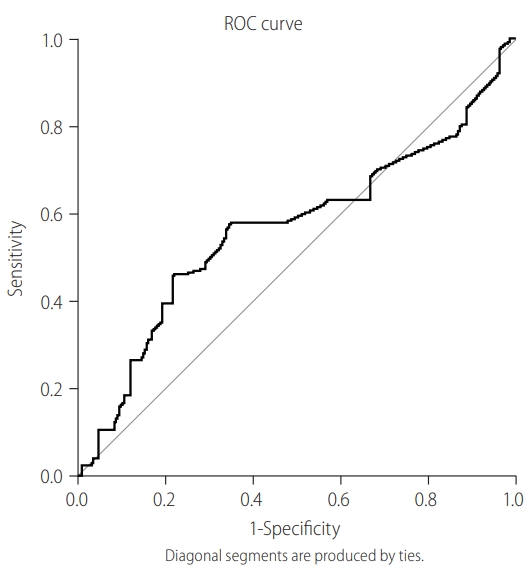
To determine the cut-off value of IgM anti-HBc as a sensitive marker of AHB, ROC was plotted. Figure 1 shows AUROC (0.87) using the sensitivity of IgM anti-HBc at various cut-off points. The sensitivity and specificity for the cut-off value of 20.5 were 93.3% and 92.7%, respectively, while PPV and NPV at this cut-off were 86.9% and 95.9%, respectively. The diagnostic performance of various S/Co ratios of IgM anti-HBc was evaluated based on the distribution of patients in each group (Table 4). IgM anti-HBc S/Co ratios of <10 and >30 were found to be significant markers for CHB-AE and AHB, respectively ( P <0.001). To determine the cutoff value of INR as a sensitive marker of AHB, ROC was plotted. Figure 2 shows AUROC (0.56) using the sensitivity of INR at various cut-off points. The sensitivity and specificity for the cut-off value of 1.27 were 57.9% and 45.1%, respectively. After combining these two markers, AUROC slightly increased to 0.90, while sensitivity decreased to 80.2% and specificity remained almost the same (92.1%; Fig. 3). These results showed that combining INR to IgM anti-HBc did not improve its predicting capability as an independent marker.
Receiver operating characteristic curve (ROC) plotted to determine the cut-off value of IgM anti-HBc as a sensitive marker of AHB. Figure shows the area under the ROC curve (AUROC) using the sensitivity of IgM anti-HBc at various cut-off points. IgM anti-HBc, immunoglobulin M antibody to hepatitis B core antigen; AHB, acute hepatitis B.
Receiver operating characteristic curve (ROC) plotted to determine the cut-off value of international normalized ratio (INR) as sensitive marker of acute hepatitis B. Figure shows the area under the ROC curve (AUROC) using the sensitivity of INR at various cut-off points.
Diagnostic value of HBV DNA levels for the differentiation between AHB and CHB-AE
Although the difference between the two groups for log10 HBV DNA values was not statistically significant, ROC was plotted to understand the role of HBV DNA levels as well as to find out the cut-off value of HBV DNA levels as a predictor of AHB. AUROC using log10 HBV DNA at various cut-off points was calculated. A cut-off value of ≤10.5 log10 IU/mL could predict AHB with a sensitivity of 51.3%, specificity of 51.4%, and PPV of 50.7. HBV DNA viral load was not significantly different between the two groups.
Role of combining virological markers in the differentiation between AHB and CHB-AE
The diagnostic efficacy of serological markers, such as qHBsAg, HBeAg, and HBV DNA, in combination with IgM anti-HBc at their respective cut-off points was analyzed. After combining a cut-off of 10.5 log10 IU/mL for HBV DNA and 2.7 log10 IU/mL for qHBsAg, the sensitivity increased from 97.8% to 98.9% while the specificity significantly decreased from 51.8% to 49.1%, as compared to using IgM anti-HBc alone. Similarly, by combining HBeAg S/Co (cut-off value of 1.5), the specificity also decreased (13.8%). These results showed that combining virological markers did not prove to be a better indicator to differentiate AHB from CHB-AE, as compared to using IgM anti-HBc alone.
DISCUSSION
The present study was performed to understand the role of various virological markers in differentiating AHB from CHB-AE. Both AHB and CHB-AE resemble each other in clinical presentation as well as in terms of biochemical characteristics. For differentiating this scenario on initial presentation, appropriate clinical history of the patients is required to highlight the onset of symptoms, of which is often lacking and thus isn’t helpful in many cases [14]. For numerous decades, the qualitative estimation of serological markers of hepatitis B infection has been the mainstay of diagnosis, in addition to the evaluation of AST and HBV DNA. Qualitative estimation using old enzyme immunoassays, which are usually standardized at a higher threshold values, lack the sensitivity to detect the variable at lower concentrations; furthermore, AST values lack specificity that represents the state of hepatic necrosis only [15]. For many years, IgM anti-HBc was considered a specific marker for AHB due to the old assays that were standardized at 600–700 PEI (Paul-Ehrlich Units) or avidity index, which detected only high values. However, with the help of the latest diagnostic techniques like chemiluminescence, quantitation of this parameter on well-characterized specimens of acute and CHB revealed a considerable percentage of positive cases among chronic hepatitis cases, but with lower levels. This has been demonstrated in various studies, as shown in Table 5 [16-20].

Comparative analysis of various studies evaluating the role of serology for differentiating between AHB and CHB-AE
In our study, the median levels of IgM anti-HBc and INR were found to be significantly different between the two groups, and also proven to be a sensitive predictor for AHB group. The result of the present study is in accordance with previous studies, which used different methods of quantification for IgM anti-HBc against semiquantitative serology [16-20]. Higher IgM anti-HBc titers suggest a highly active immune response which promotes B cell differentiation into IgM producing plasmablasts. Since our hospital is a tertiary care liver institute, the presentation of majority cases in early acute phase of the illness can be responsible for the higher cut-off value obtained in our study, or it can also be due to the inherent immunological difference by region. The serum level of INR, which reflect the degree of coagulopathy in a patient with hepatic disease, was an independent predictor for AHB, although it had a lesser sensitivity compared to IgM anti-HBc. This result could not be inferred in isolation of other coagulation parameters such as platelets, prothrombin time, and activated partial thromboplastin time. We could not follow up with the patients for evaluating the dynamic values of INR using the prescribed drug regimen. Further studies are required to confirm our findings.
The present study did not find any significant differences between HBV DNA levels of both groups. This was in contrast to other studies which reported higher levels of HBV DNA being associated with CHB-AE as compared to AHB [19-21]. HBV DNA levels in CHB-AE group in our study were quite comparable to those of AHB group. This could be due to the non-immunosuppressed state of the subjects, rapid immune clearance following HBV reactivation, effect of antiviral treatment which suppresses viral replication, and misallocation of some subjects in the two groups, as they were not fully clinically assessed.
HBeAg positivity has been found more frequently in patients with acute infection than in those with chronic infection, but the difference is not statistically significant [22,23]. The present study showed no significant difference between the values of HBeAg in the two groups. Recent data support the fact that high levels of HBsAg are associated with viral replication and disease activity. In acute hepatitis, the levels of HBsAg are generally above 1×107 IU/L, and they decrease in the recovery phase [24-26]. Although a median value of qHBsAg (4.12 log10 IU/mL) was higher in AHB than in CHB-AE (1.09 log10 IU/mL) in the present study, the association was not statistically significant. Various studies have reflected significantly higher values of qHBsAg for acute phase [16].
The liver fibrosis status in the two groups was not compared using Fibroscan, FIB-4 index, and M2BPGi. However, the APRI score and AST/ALT ratio did not differ between the two groups. While AST/ALT ratio >1 is recommended as a predictor of cirrhosis and has sensitivity and specificity of 81.3% and 55.3%, respectively, an APRI of more than 1.5 has AUROC of 80% and 89%, respectively, for advanced fibrosis F3–F4 and cirrhosis [27-29]. Numerous studies have highlighted that the data concerning the clinical utility of transient elastography in hepatitis B appears promising with 84% and 65% positive and NPVs, respectively, for a cut-off of 7.0 kPa. Although acute hepatitis can produce false-positive results in the APRI, Forns index, FIB-4, or Fibrometer tests, which all measure the levels of aminotransferases, not much is present in the existing literature on differentiating AHB from CHB-AE using these markers [30].
Although a few studies have highlighted the importance of combining IgM anti-HBc and HBV DNA to increase the sensitivity and specificity of newly created marker, the present study could not find such association [18]. Although an increase in sensitivity was seen after combining IgM anti-HBc with qHBsAg, HBV DNA, and HBeAg, the specificity decreased remarkably. This could be due to the fact that not all of the subjects in the study underwent testing for all serological markers during follow-up visits to the hospital.
In conclusion, CHB-AE causing derangement of liver functions may be seen in a flare of HBV during the immune clearance phase or HBV reactivation in patients with inactive hepatitis infection or resolved HBV infection. In endemic countries like India, the differentiation between CHB-AE and AHB is important for both prognostication and management of the disease. Quantitation of IgM anti-HBc can work as a simple marker of differentiation between AHB and CHB-AE. Our analysis of various serological markers in this study showed that only IgM anti-HBc was a significant discriminating factor between CHB-AE and AHB, and that combining other markers did not add to its discriminating power.
Study limitations
The present study had some limitations due to its retrospective nature, and it was purely dependent on the patient information that were available in the hospital system. To find the best possible operational and feasible approach, the patients were retrospectively categorized into two groups based on their clinical history as well as the presence or absence of HBsAg antigen after 6 months [6,21,31]. Also, the possibility of misclassification of cases cannot be negated, as the loss of HBsAg at exactly 6 months is not very commonly reported. Therefore, further studies are needed to highlight the role of serological markers in differentiating AHB and CHB-AE. Furthermore, the evaluation of cut-off of IgM anti-HBc by quantitative assay is recommended over the semiquantitative one used in this study for better statistical agreement, and INR results on serial follow-up would add more to our knowledge.
Abbreviations
AHB
acute hepatitis B
ALT
alanine aminotransferase
anti-HBe
antibody to HBeAg
APASL
Asian Pacific Association for the Study of the Liver
APRI
AST platelet ratio index
AST
aspartate aminotransferase
AUROC
areas under ROC
CHB-AEs
acute exacerbations in chronic hepatitis B
CLIA
Chemiluminescence Immunoassay
HBeAg
hepatitis B e antigen
HBsAg
hepatitis B surface antigen
HBV
hepatitis B virus
IgM anti-HBc
immunoglobulin M antibody to the hepatitis B core antigen
INR
international normalized ratio
NPV
negative predictive value
PEI
Paul-Ehrlich Units
PPV
positive predictive value
qHBsAg
quantitative HBsAg
RLUs
relative light units
ROC
receiver operating characteristic
S/Co
signal cut-off
SPSS
Statistical Package for the Social Sciences
Notes
Author contributions
Ekta Gupta: Conception, designing, and supervision of work. Writing and revision of manuscript
Sujata Lall, Pragya Agarwala: Designing, writing, and revision of manuscript
Guresh Kumar: Statistical analysis and interpretation of data
Manoj Kumar Sharma: Critical revision of the manuscript
Conflict of Interest
The authors have no conflicts of interest to disclose.
References
Article information Continued
Notes
Study Highlights
This study investigated whether quantitative serology is valuable in differentiating acute hepatitis B (AHB) from chronic hepatitis B with acute exacerbation (CHB-AE). A total of 172 patients were included and divided into two groups: AHB (89, 51.7%) and CHB-AE (83, 48.3%). The median value of signal by cut-off (S/Co) ratio of IgM anti-HBc was significantly higher (30.44 vs. 8.63), and the mean value of INR was significantly lower (1.62±0.17 vs. 1.88±1.24) in AHB group compared to CHB-AE group (all P<0.05). The results of this study suggest that quantitative serologic markers could be helpful in the diagnosis of patients with HBV infection.